
Preparing for a challenging assessment requires careful attention to various key topics and mastering essential concepts. In this section, you’ll find valuable insights and resources to help you tackle the material with confidence. Focus on understanding fundamental principles, applying formulas, and reviewing critical ideas that will be tested.
Focused preparation is key to success. By reviewing relevant topics, practicing problem-solving techniques, and reinforcing your understanding of core principles, you will be able to approach the test with clarity and efficiency. This guide will help you identify areas of strength and pinpoint those that may need additional study.
Mastery of concepts is the goal. With strategic practice and time management, you’ll be able to maximize your performance and handle any question that comes your way. Use the following sections to guide your study process and achieve your best possible results.
Chemistry Honors Semester 1 Exam Review Answers

As you approach your upcoming assessment, it is essential to consolidate your knowledge and identify the core principles that will be tested. This section provides a breakdown of crucial topics and offers guidance on how to prepare effectively. With a focus on key concepts, problem-solving techniques, and essential equations, you’ll be able to approach the test with greater confidence.
Master Key Concepts
Understanding the foundational ideas in the subject is vital. From atomic structure to chemical reactions, reviewing these concepts will form the basis of your preparation. Focus on the relationships between various elements and compounds, as well as how these interact in different scenarios. The more familiar you are with these building blocks, the easier it will be to apply them during the assessment.
Practice Problem-Solving Techniques

Application of knowledge is just as important as understanding the theory. Practice solving problems related to stoichiometry, balancing equations, and molecular interactions. Being able to quickly work through calculations and demonstrate your understanding of chemical processes will make a significant difference in your performance. Make sure to go through sample questions and familiarize yourself with common problem formats.
Consistent practice will sharpen your skills and help you recall information more quickly during the test. The more problems you solve, the more confident you’ll become in tackling any question on the day of the assessment.
Key Concepts to Focus On
When preparing for any rigorous assessment, it is important to focus on the most vital principles that will form the foundation of the test. These core ideas are often revisited throughout the material and require a deep understanding to ensure success. Mastery of these concepts will not only help with direct questions but also provide the skills to apply your knowledge in different scenarios.
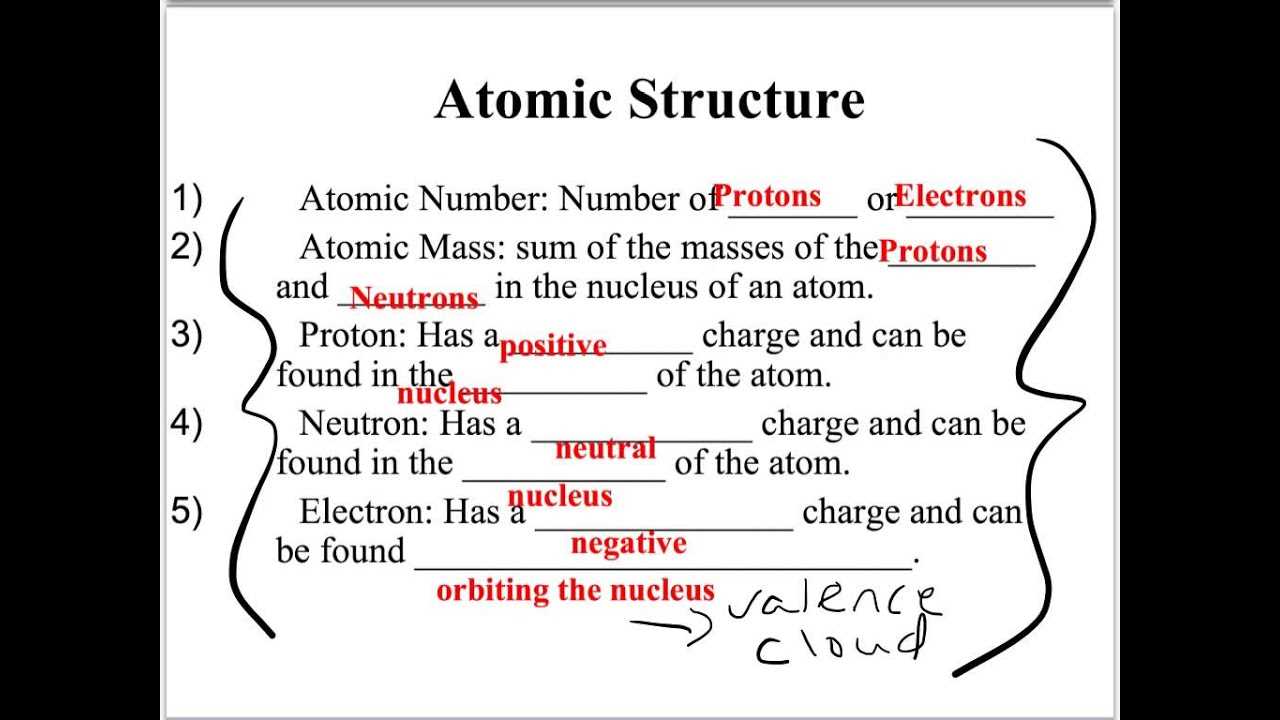
Understanding Atomic and Molecular Structures
The building blocks of matter are fundamental to almost every topic in the subject. Understanding atomic structure, electron configurations, and the behavior of molecules is essential. These concepts are the basis for more complex subjects, including chemical reactions and bonding theories. Reviewing the periodic table and recognizing patterns in atomic size, electronegativity, and ionization energy will help you navigate various questions related to element interactions.
Principles of Chemical Reactions
Being able to identify and balance chemical reactions is critical. Focus on recognizing reaction types such as synthesis, decomposition, combustion, and redox reactions. Learn how to predict the products of a reaction and understand how conservation of mass applies in different situations. Pay attention to the concepts of activation energy and reaction rates, as these are frequently tested topics.
Important Formulas to Remember
Mastering key mathematical relationships is crucial for success in any scientific field. These formulas form the basis for solving problems and applying theoretical knowledge to practical scenarios. Whether it’s calculating quantities, determining reaction rates, or analyzing molecular behavior, being familiar with these essential equations will significantly improve your ability to solve questions effectively.
Key Mathematical Relationships
Below are some of the most important formulas that you should be able to recall during your preparation:
- Ideal Gas Law: PV = nRT – This equation relates the pressure, volume, temperature, and amount of gas in a system.
- Molality: m = moles of solute / kg of solvent – Used to express the concentration of a solution.
- Density: ρ = mass / volume – Essential for determining the density of a substance.
- Avogadro’s Number: 6.022 × 10²³ – The number of particles in one mole of a substance.
- Boyle’s Law: P₁V₁ = P₂V₂ – Describes the inverse relationship between pressure and volume at constant temperature.
Reaction and Energy Calculations
In addition to basic physical formulas, understanding the mathematical principles behind chemical reactions is critical. Here are some essential equations:
- Concentration (Molarity): M = moles of solute / liters of solution – Used for determining the concentration of a solution in mol/L.
- Heat Transfer: q = mcΔT – Calculates the amount of heat energy required to change the temperature of a substance, where m is mass, c is specific heat, and ΔT is the change in temperature.
- Law of Conservation of Mass: mass reactants = mass products – This principle underlies the balancing of chemical equations.
Familiarity with these formulas will help you solve a wide range of problems and apply scientific principles with ease. Make sure to practice these equations until they are second nature to you.
Types of Chemical Reactions Covered
Understanding the various forms of reactions is essential for solving problems related to molecular interactions and transformations. These reactions represent the fundamental processes by which substances are altered, and recognizing the type of reaction is key to predicting outcomes and balancing equations. Here, we will focus on the most common categories of reactions and their characteristics.
Classification of Reactions
The following table outlines the different reaction types you should be familiar with, along with their defining characteristics and examples:
| Reaction Type | Characteristics | Example |
|---|---|---|
| Synthesis | Two or more reactants combine to form one product. | 2H₂ + O₂ → 2H₂O |
| Decomposition | A single reactant breaks down into two or more simpler products. | 2H₂O → 2H₂ + O₂ |
| Single Replacement | One element replaces another in a compound. | Zn + CuSO₄ → ZnSO₄ + Cu |
| Double Replacement | Two compounds exchange components to form two new compounds. | NaCl + AgNO₃ → NaNO₃ + AgCl |
| Combustion | A substance reacts with oxygen, often producing heat and light. | CH₄ + 2O₂ → CO₂ + 2H₂O |
| Redox | Involves the transfer of electrons between substances. | 2Na + Cl₂ → 2NaCl |
Important Notes
Recognizing the type of reaction helps in predicting the products and balancing the equation. Make sure to practice identifying and classifying different reactions during your preparation, as this knowledge is critical for problem-solving and applying concepts effectively during the assessment.
Periodic Table Trends and Patterns
The arrangement of elements in the periodic table reveals distinct trends and behaviors that govern their physical and chemical properties. These patterns help predict how elements will interact, react, and combine with others. Understanding these trends is crucial for grasping the underlying principles of element behavior and making sense of their interactions in various contexts.
Key Trends Across the Table

Several key trends can be observed across the rows (periods) and columns (groups) of the periodic table. These trends provide insight into the characteristics of elements, such as their size, electronegativity, and ionization energy:
- Atomic Radius: The size of an atom decreases as you move from left to right across a period, but increases as you move down a group.
- Electronegativity: Elements tend to have higher electronegativity as you move from left to right across a period and lower electronegativity as you move down a group.
- Ionization Energy: The energy required to remove an electron increases across a period and decreases down a group.
Group Characteristics
Elements within the same group share certain similarities due to their similar electron configurations. For example:
- Alkali Metals (Group 1): These elements are highly reactive, especially with water, and have a single electron in their outer shell.
- Halogens (Group 17): These elements are highly reactive and tend to form salts when combined with metals.
- Noble Gases (Group 18): Known for their stability and low reactivity, these elements have full outer electron shells.
Recognizing these trends not only enhances your understanding of the periodic table but also enables you to predict the behavior of elements in chemical reactions and bonding scenarios.
Balancing Chemical Equations Tips
Balancing chemical reactions is a fundamental skill in understanding how matter interacts and transforms. It ensures that the number of atoms of each element is conserved, in accordance with the law of conservation of mass. Mastering the techniques for balancing equations can simplify complex problems and enhance your overall understanding of chemical processes.
Step-by-Step Approach
To effectively balance an equation, follow these steps:
- Write the unbalanced equation: Start with the correct chemical formulas for all reactants and products.
- Balance one element at a time: Begin with elements that appear in only one reactant and one product. Adjust coefficients as necessary.
- Balance hydrogen and oxygen last: These elements often appear in multiple compounds, so they should be left until the end to avoid unnecessary changes.
- Check your work: After adjusting all coefficients, verify that the number of atoms on both sides is equal for each element.
Common Tips for Success
- Start with complex molecules: If a compound contains multiple elements, balance it before simpler molecules to avoid complications.
- Use fractional coefficients if needed: If you encounter fractional values, multiply all coefficients by the denominator to eliminate fractions.
- Double-check atom counts: Always ensure that each element is balanced before moving on to the next step.
- Practice, practice, practice: Balancing equations becomes easier with experience, so work through numerous examples to develop fluency.
With persistence and practice, balancing chemical equations will become a more intuitive and less time-consuming task, allowing you to approach problems with confidence.
Acids and Bases Review Guide
Understanding the properties and behaviors of acids and bases is crucial for studying various chemical reactions and their applications. These substances play a significant role in a wide range of processes, from neutralization reactions to pH regulation in biological systems. By grasping the basic principles and characteristics of acids and bases, you can predict their interactions and understand their impact on chemical equilibria.
Acids are substances that tend to donate protons (H⁺ ions), while bases are substances that accept protons or donate hydroxide ions (OH⁻). The strength of an acid or base is determined by its ability to dissociate in water, and this concept is essential when evaluating their reactivity and role in chemical reactions.
When working with acids and bases, it’s important to understand key concepts such as the pH scale, acid-base titrations, and neutralization reactions. Mastery of these topics is fundamental to solving problems and predicting the outcomes of chemical interactions involving acidic and basic substances.
Stoichiometry and Molar Calculations

Understanding the relationship between the quantities of reactants and products in a chemical reaction is fundamental to solving many types of problems in chemistry. These calculations, which involve converting between moles, masses, and volumes, are essential tools for predicting the amounts of substances involved in reactions. Mastering stoichiometry and molar conversions allows you to accurately calculate yields and determine the efficiency of chemical processes.
In this section, we’ll focus on the key methods used in stoichiometric calculations, such as mole-to-mole conversions, using molar masses, and applying ideal gas laws for reactions involving gases. These calculations are based on the principle that atoms are conserved in a reaction, so the amount of each substance is proportional to its coefficient in the balanced equation.
Stoichiometric Calculation Steps
Here’s an overview of how to approach stoichiometric problems:
- Write the balanced equation: Ensure that the equation is properly balanced, as this will dictate the mole ratios of the reactants and products.
- Convert units to moles: If given quantities in grams, liters, or molecules, convert them into moles using the molar mass or ideal gas laws as necessary.
- Use mole ratios: Apply the coefficients from the balanced equation to convert moles of one substance to moles of another.
- Convert moles to desired units: Finally, convert your result from moles to the required units (e.g., grams, liters, molecules) using molar mass or other relevant conversion factors.
Example Problem: Molar Mass and Moles
Let’s look at an example of a simple stoichiometric calculation:
| Substance | Molar Mass (g/mol) | Given Quantity (g) | Moles |
|---|---|---|---|
| Water (H₂O) | 18.02 | 36.04 | 36.04 / 18.02 = 2 moles |
This is just one of many examples of how stoichiometry and molar calculations are used to determine the amount of a substance in a chemical process. With practice, these calculations become a powerful tool for understanding and predicting the behavior of substances in chemical reactions.
Thermodynamics: Key Principles to Know
The study of energy and its transformations is essential for understanding how physical and chemical processes occur. Whether it’s the conversion of heat into work or the flow of energy in a system, the fundamental laws governing energy changes play a crucial role in every reaction. A strong grasp of thermodynamics helps explain why certain reactions happen spontaneously while others require an external source of energy.
In this section, we will explore the core principles that govern energy interactions within systems, focusing on concepts such as internal energy, enthalpy, and entropy. These principles are not only fundamental to theoretical studies but also have practical applications in fields like engineering, biology, and environmental science.
First Law of Thermodynamics
The first law, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transferred or transformed. This principle means that the total energy of an isolated system remains constant. In chemical reactions, energy may be absorbed or released as heat, but the overall energy balance stays the same.
Second Law of Thermodynamics
The second law introduces the concept of entropy, a measure of disorder in a system. This law asserts that in any energy transfer or transformation, the total entropy of a system and its surroundings always increases. Essentially, energy will spontaneously disperse to increase disorder, making some processes irreversible without external intervention.
Understanding these principles allows you to predict how energy flows in different systems and anticipate the direction of natural processes. The relationship between energy changes and entropy is particularly important for determining the spontaneity of reactions, as some processes naturally increase entropy, while others require work to proceed.
Understanding Chemical Bonding Basics

The way atoms interact and form connections is fundamental to understanding the structure and properties of matter. When atoms come together to form molecules, they do so by sharing or transferring electrons in a process that leads to the formation of chemical bonds. These interactions determine the physical and chemical properties of substances, from their boiling points to their reactivity with other compounds.
In this section, we will explore the basic types of bonds that form between atoms and how these bonds influence the characteristics of compounds. The two primary types of bonds are ionic bonds and covalent bonds, each with its unique properties and formation mechanisms. By understanding these fundamental concepts, you can better comprehend the behavior of various materials in chemical reactions.
Types of Chemical Bonds
The two main categories of chemical bonds are:
- Ionic Bonds: Formed when electrons are transferred from one atom to another, creating charged particles (ions) that attract each other due to electrostatic forces.
- Covalent Bonds: Occur when two atoms share electrons to achieve a more stable electron configuration, typically between nonmetals.
Bond Strength and Properties

The strength and properties of a bond depend on the elements involved and the nature of the bond itself:
- Ionic Bonds: Tend to have high melting and boiling points, as the strong attraction between ions requires a lot of energy to break.
- Covalent Bonds: Can vary in strength, from very weak bonds like those in gases to strong bonds in solids like diamonds, depending on how electrons are shared.
Understanding these types of bonds and their properties is crucial for predicting how substances will behave in various conditions and reactions. This knowledge also lays the foundation for more advanced concepts, such as molecular geometry and reaction mechanisms.
Lab Techniques and Safety Protocols
Successful experimentation relies not only on understanding theoretical concepts but also on the proper execution of techniques in the laboratory. Mastering laboratory procedures ensures that experiments are conducted accurately and efficiently while minimizing errors. Equally important are the safety protocols that protect individuals from potential hazards, ensuring a safe working environment for all involved.
In this section, we will cover essential techniques and safety measures you need to understand to work effectively in a lab setting. Proper handling of equipment, the use of protective gear, and knowing how to respond in case of an emergency are all crucial components of a productive and safe laboratory experience.
Essential Lab Techniques
Here are some basic techniques that every laboratory worker should be familiar with:
- Measuring and Mixing: Accurate measurement of liquids, solids, and gases is essential for consistent results. Be sure to use the correct instruments, such as pipettes, burettes, and balances, to achieve precision.
- Heating and Cooling: Understanding the correct temperatures for various reactions and knowing how to safely heat or cool substances is critical to prevent accidents or errors.
- Filtration and Separation: Techniques like filtration, distillation, and centrifugation help separate mixtures and isolate specific components for analysis.
Lab Safety Protocols
Safety is paramount in any laboratory. Always follow these protocols to minimize the risk of injury or exposure to harmful substances:
- Protective Equipment: Always wear appropriate protective gear, such as safety goggles, gloves, and lab coats, to safeguard against spills, splashes, or flying debris.
- Proper Ventilation: Ensure proper ventilation when working with volatile chemicals to avoid inhaling toxic fumes or gases.
- Know Emergency Procedures: Be aware of emergency exits, fire extinguishers, eyewash stations, and first aid kits. Familiarize yourself with the steps to take in case of an accident or chemical spill.
By mastering these techniques and adhering to safety protocols, you ensure that both you and your surroundings remain safe while conducting scientific investigations. Lab success depends not only on what you know but how you apply that knowledge in a controlled, responsible manner.
Common Mistakes to Avoid in Exams
When preparing for any assessment, it’s easy to make simple mistakes that can cost valuable points. These errors often stem from a lack of attention to detail, poor time management, or misunderstanding the requirements of the questions. Identifying and avoiding common pitfalls can significantly improve performance and help you avoid unnecessary mistakes during your next test.
In this section, we will discuss several frequent errors students make and provide tips on how to avoid them. By being aware of these issues and practicing strategies to counteract them, you can enhance your chances of achieving better results and approach your assessments with more confidence.
Not Reading Instructions Carefully

One of the most common mistakes is failing to fully read and understand the instructions. Often, students rush through the beginning of a test, missing important details that could affect their responses. To avoid this:
- Take time to read every instruction thoroughly.
- Highlight key information, such as word limits or specific formats requested.
- If unsure about an instruction, ask for clarification before starting the section.
Mismanaging Time
Another frequent error is not managing time effectively. Spending too much time on one question can leave insufficient time for others. To overcome this:
- Prioritize questions based on their value and complexity.
- Set time limits for each section and stick to them as much as possible.
- Move on if you’re stuck, and come back to challenging questions later if time allows.
Not Reviewing Work
Failing to review answers before submitting is another common mistake. Even if you feel confident about your responses, it’s crucial to double-check your work for errors. To prevent this:
- Leave a few minutes at the end of the test to review your answers.
- Check for common errors, such as miscalculations or incomplete responses.
- Look for any missed instructions or questions that were skipped accidentally.
By avoiding these mistakes and preparing with a focused strategy, you can significantly improve your performance and feel more prepared going into any assessment. A careful approach and a bit of practice can make all the difference.
Practice Problems for Quick Review
One of the most effective ways to reinforce your knowledge and solidify your understanding of key concepts is through practice. Working through problems allows you to apply what you’ve learned and identify areas where further clarification may be needed. In this section, you’ll find a set of practice questions designed to test your knowledge and help you prepare more effectively for any upcoming assessments.
These problems cover a range of topics and difficulty levels, allowing you to focus on the areas that are most relevant to your preparation. By working through these questions, you will not only strengthen your problem-solving skills but also boost your confidence for any upcoming tests or evaluations.
Problem Set 1: Balancing Chemical Equations
Complete the following equations by balancing them:
- H2 + O2 → H2O
- C3H8 + O2 → CO2 + H2O
- Na + Cl2 → NaCl
Problem Set 2: Molar Calculations
Answer the following questions based on molar relationships:
- How many moles are in 50 grams of H2O? (Molar mass of H2O = 18.015 g/mol)
- Calculate the number of atoms in 2 moles of NaCl.
- Determine the mass of 3 moles of CO2 (Molar mass of CO2 = 44.01 g/mol).
Problem Set 3: Acid-Base Reactions
Write the products of the following reactions:
- HCl + NaOH →
- H2SO4 + KOH →
- HNO3 + Ca(OH)2 →
By practicing these problems, you will reinforce your understanding of key concepts and improve your ability to apply them in different scenarios. Make sure to carefully review your answers and identify any areas where you may need further clarification or practice.
How to Study for This Exam Effectively
Preparing for a challenging assessment requires a structured approach and careful planning. To maximize your chances of success, it is essential to engage with the material in a way that allows you to understand, retain, and apply key concepts. In this section, we will explore practical strategies to help you study more efficiently and improve your performance.
Effective studying involves more than just reading through notes or textbooks. It requires active engagement with the material, practicing key skills, and ensuring that you are comfortable with various types of questions that may appear on the test. Creating a study plan, organizing your materials, and regularly reviewing content are all crucial steps in the process.
Study Plan and Time Management
Creating a study schedule is the first step in preparing for any assessment. This helps you allocate time to each topic based on its importance and difficulty level. Divide your study sessions into manageable blocks and make sure to take breaks to keep your mind fresh. Below is an example study plan:
| Day | Study Focus | Time Allotted |
|---|---|---|
| Monday | Review key formulas and equations | 2 hours |
| Tuesday | Practice problem-solving techniques | 2 hours |
| Wednesday | Focus on key concepts in reactions | 2 hours |
| Thursday | Mock tests and self-assessment | 2 hours |
| Friday | Review weak areas and final practice | 2 hours |
Active Learning Techniques
Simply reading through your notes might not be enough. To ensure better retention and understanding, try using active learning techniques such as:
- Practice problems: Working through examples is one of the most effective ways to reinforce learning and identify areas for improvement.
- Teach someone else: Explaining concepts to a peer can help solidify your understanding and reveal any gaps in your knowledge.
- Flashcards: These are great for memorizing key terms, formulas, and reactions quickly.
- Self-testing: Take mock tests under timed conditions to simulate the actual assessment environment.
By following a structured study routine, engaging in active learning, and using the right resources, you can optimize your preparation and approach your assessment with confidence.
Tips for Managing Exam Stress
Feeling stressed before a major test is a common experience. However, managing this pressure effectively can make a significant difference in your performance. Learning how to stay calm and focused under pressure is crucial for not only performing well but also maintaining your well-being throughout the study process.
In this section, we’ll explore some strategies to help you manage stress before, during, and after an important assessment. By implementing these tips, you can create a more balanced and productive study environment, reducing anxiety and increasing your chances of success.
Preparation is Key
One of the main causes of stress is feeling unprepared. Creating a clear plan and sticking to it will give you a sense of control over your studies. Here are some steps to take:
- Start early: Begin your study sessions well in advance to avoid last-minute cramming, which increases stress levels.
- Break tasks into smaller chunks: Instead of trying to study everything at once, divide your tasks into smaller, manageable sections.
- Set realistic goals: Create specific, achievable goals for each study session to keep you on track.
Relaxation Techniques

Even with the best preparation, stress can still build up. Incorporating relaxation techniques into your routine can help you stay calm and focused. Try the following practices:
- Deep breathing: Practicing deep breathing exercises can lower heart rates and reduce anxiety.
- Mindfulness or meditation: Taking just a few minutes each day for mindfulness or meditation can help clear your mind and relieve stress.
- Physical activity: Regular exercise, even a short walk, helps release tension and improves concentration.
Sleep and Nutrition
Maintaining good physical health is essential during stressful times. Never underestimate the importance of sleep and a balanced diet:
- Get enough sleep: A good night’s rest enhances focus, memory, and overall well-being.
- Eat nutritious meals: Choose foods that boost brain function, such as those rich in omega-3 fatty acids and antioxidants.
- Stay hydrated: Drink plenty of water to keep your body and mind functioning at their best.
Positive Thinking
Staying positive can help shift your mindset from anxiety to confidence. Replace negative thoughts with more positive affirmations:
- Focus on progress, not perfection: Celebrate small victories along the way to boost morale.
- Visualize success: Imagine yourself performing well and handling the test confidently.
- Talk to someone: Sharing your concerns with a friend or family member can relieve stress and provide valuable support.
By implementing these stress-management techniques, you can approach any upcoming challenges with a calm and focused mindset. Managing stress is not only about performing well but also about taking care of your mental and physical health throughout the entire process.
Sample Questions with Detailed Answers

Understanding how to approach different types of questions is crucial for performing well in assessments. In this section, we’ll explore several sample problems, followed by comprehensive explanations. These examples will guide you through problem-solving techniques, helping you better grasp the key concepts and enhance your critical thinking skills.
Question 1: Balancing a Chemical Reaction
Problem: Balance the following reaction:
C_4H_{10} + O_2 → CO_2 + H_2O
Solution: To balance this reaction, start by balancing the elements one by one. Begin with carbon (C), then move to hydrogen (H), and finally oxygen (O). The process will involve adjusting coefficients to ensure the same number of atoms of each element on both sides of the equation.
- First, balance carbon by placing a coefficient of 4 in front of CO₂: C₄H₁₀ + O₂ → 4CO₂ + H₂O
- Next, balance hydrogen by placing a coefficient of 5 in front of H₂O: C₄H₁₀ + O₂ → 4CO₂ + 5H₂O
- Now, balance oxygen. There are 13 oxygen atoms on the right side (4 from CO₂ and 5 from H₂O). Place a coefficient of 13/2 in front of O₂: C₄H₁₀ + 13/2 O₂ → 4CO₂ + 5H₂O
- To eliminate the fraction, multiply all coefficients by 2: 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
Final balanced equation: 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
Question 2: Mole-to-Mole Conversion
Problem: How many moles of O₂ are required to completely react with 3 moles of C₄H₁₀ (butane)?
Solution: To solve this, use the coefficients from the balanced equation to find the mole ratio between butane and oxygen. From the balanced equation 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O, the ratio of C₄H₁₀ to O₂ is 2:13.
- Set up a proportion: (3 moles of C₄H₁₀) × (13 moles of O₂ / 2 moles of C₄H₁₀) = 19.5 moles of O₂
Final result: 19.5 moles of O₂ are needed to react with 3 moles of C₄H₁₀.
These types of problems can be encountered in various scenarios, and understanding the steps to solve them will improve your problem-solving skills and your overall performance in assessments.
Strategies for Time Management During the Test
Efficient use of time is critical when facing a timed assessment. Having a solid approach to managing the available time can make a significant difference in how well you perform. In this section, we’ll cover some practical techniques to ensure you can work through all sections effectively and avoid unnecessary stress.
- Prioritize the Questions: Start by quickly scanning the entire test. Identify questions that seem easier or familiar and tackle them first. This helps build confidence and ensures you don’t miss out on simpler points.
- Allocate Time per Section: Divide the total time by the number of sections or questions. Set a time limit for each part and stick to it. If you find yourself spending too much time on one question, move on to the next and return to it later.
- Read Instructions Carefully: Sometimes, the instructions contain crucial details that can help save time or guide you to the right approach. Reading them thoroughly, even if it seems tedious, can prevent mistakes.
- Don’t Get Stuck on One Question: If a question is taking too long to answer, it’s better to leave it and come back later rather than waste precious time. Mark it and move on to other items to ensure you’re making the best use of your time.
- Keep Track of Time: Periodically glance at the clock to assess your progress. If you’re falling behind, adjust your pace or skip a question temporarily to avoid rushing at the end.
- Review Your Work: If time allows, leave a few minutes at the end to go over your answers. Check for simple mistakes, and make sure your responses are complete and clear.
By incorporating these strategies, you can improve your ability to manage the allotted time effectively, reduce anxiety, and increase your chances of performing well under pressure.