| Reaction Mechanisms |
Stepwise processes and catalysts |
Interpreting sequences and analyzing efficiency |
How to Approach Free Response Questions
Tackling open-ended questions requires a strategic mindset and a thorough understanding of the subject matter. These tasks are designed to test not only your grasp of key principles but also your ability to apply them effectively in varied scenarios.
Begin by carefully analyzing what is being asked. Look for keywords that indicate the type of answer expected, such as calculations, explanations, or diagrams. Breaking down the question into smaller parts can help you focus on addressing each component systematically.
Clear and concise answers are crucial. Use logical steps to present your solutions, ensuring that your reasoning is well-supported by evidence or formulas where necessary. Avoid unnecessary details that might distract from the main points.
Finally, review your work to check for accuracy and completeness. Verifying units, equations, and terminology ensures that your responses demonstrate both precision and a deep understanding of the material.
Scoring Guidelines for the 2009 Exam
The grading criteria for this assessment are designed to evaluate a student’s mastery of key principles, logical reasoning, and the ability to apply concepts effectively. Scoring is based on a combination of accuracy, clarity, and depth of understanding demonstrated in each response.
Key Elements of the Scoring Process
Each part of the evaluation is scored with specific guidelines in mind to ensure consistency. These key factors typically include:
- Correctness: Accurate application of concepts, calculations, and formulas.
- Logical Structure: Clear organization and systematic approach in presenting solutions.
- Conceptual Understanding: Ability to explain reasoning and demonstrate a deep grasp of the material.
Maximizing Your Score
To achieve the best possible score, it is important to follow certain strategies when answering each question. Consider these tips:
- Carefully read each question to fully understand what is being asked before you begin.
- Provide step-by-step work and explanations, especially for complex problems.
- Use precise language and appropriate units, ensuring that your work is easily understandable.
- Ensure your reasoning is clearly explained and linked to the provided information or problem context.
By adhering to these principles and focusing on structured, clear responses, you can maximize your score and demonstrate a thorough understanding of the material.
Common Mistakes to Avoid
During the evaluation, students often make several recurring mistakes that can impact their performance. Being aware of these common errors can help you avoid pitfalls and improve your chances of achieving a higher score.
One of the most frequent mistakes is not reading the question thoroughly. Skipping important details or misinterpreting what is being asked can lead to incorrect responses. It’s essential to take the time to fully understand each question before attempting to answer.
Another mistake is failing to show all work. Even if you arrive at the correct answer, not demonstrating the steps can result in losing valuable points. Scorers typically look for the logical progression of thought, so be sure to write out every step of your solution clearly.
Omitting units or using incorrect units is also a common issue. Units are a crucial part of demonstrating your understanding of the concepts, and leaving them out can make your answer incomplete. Always include the appropriate units for every calculation and measurement.
Additionally, some students may provide answers without clear explanations. Even if the calculations are correct, failing to explain your reasoning behind each step can leave your response incomplete. It’s important to articulate why you used a specific approach or equation in each case.
By avoiding these mistakes, you can present a more thorough and accurate response, leading to a higher score. Paying attention to detail, clarity, and thoroughness will enhance your overall performance on the test.
Strategies for Time Management
Effective time management is essential for success during any timed assessment. Properly allocating your time ensures that you can address each section thoroughly and avoid rushing through questions at the last minute. By utilizing a strategic approach, you can make the most of your time and improve your overall performance.
Prioritize Questions
Start by quickly scanning the entire set of questions to identify the ones you find most challenging or time-consuming. It is often wise to tackle the easier or more familiar questions first, as this allows you to build momentum and gain confidence. Save more complex problems for later, ensuring that you don’t spend excessive time on them at the outset.
Set Time Limits for Each Section

Establish a specific time limit for each question or section before starting. Allocate your time based on the weight and difficulty of the questions. For example, spend less time on shorter or more straightforward items, while giving yourself more time to address lengthy or multi-part problems. Using a watch or timer can help you stay on track and avoid spending too much time on any single part.
Taking regular, brief pauses can also help maintain focus. This will allow you to re-assess your progress and avoid mental fatigue as you work through the questions. By keeping track of time and working in a methodical manner, you increase the likelihood of completing the entire assessment to the best of your ability.
Breaking Down Complex Reactions
When faced with intricate processes or reactions, it is crucial to break them down into smaller, more manageable parts. This allows you to understand the individual steps and principles involved, making it easier to solve problems or predict outcomes. Analyzing complex scenarios step-by-step is an effective strategy for tackling challenging questions and ensuring accuracy in your work.
Start by identifying all the components involved in the reaction. This includes the reactants, products, and any intermediates or catalysts that might be present. Once you have a clear overview of the chemical species, look for patterns such as stoichiometric relationships or reaction mechanisms that can guide your next steps.
Next, focus on understanding the underlying principles that govern the reaction, such as the conservation of mass, energy, and charge. This will help you recognize how the different elements interact and lead to the formation of products. Pay attention to reaction rates, equilibrium states, and any other factors that could influence the reaction’s progression.
By breaking down each reaction into these smaller, more digestible steps, you can simplify the process and ensure a clear path to finding the correct solution. This approach will help reduce confusion and increase your confidence when handling even the most complicated chemical reactions.
Tips for Effective Answering
Answering complex questions requires clarity, structure, and a thorough understanding of the underlying concepts. To tackle each question efficiently, it’s important to approach it methodically and stay organized throughout the process. Whether it’s a theoretical inquiry or a practical problem, following a systematic approach will help you deliver precise and well-supported responses.
One of the most essential tips is to read each question carefully. Ensure that you understand what is being asked before you begin your response. Break down the question into smaller parts, identifying key terms and instructions. This will prevent you from missing any important details and ensure that you address every aspect of the question.
Next, always organize your work logically. Start with a brief introduction or a clear statement of what you’re going to discuss, followed by the necessary calculations, explanations, or steps to support your response. Present your reasoning in a step-by-step manner, showing all relevant work and justifications. This helps the reader follow your thought process and increases the chances of earning full credit.
Additionally, be mindful of the units and precision used in your calculations or explanations. Consistently applying the correct units and rounding numbers to the appropriate level of precision ensures that your responses are accurate and professional. Lastly, stay concise while being thorough–avoid unnecessary information that may distract from your main points, but ensure you cover all relevant details.
By adopting these strategies, you can answer questions more effectively and improve the quality of your responses, making sure that your understanding is clearly conveyed and well-supported.
Analyzing Sample Responses
Examining example solutions offers valuable insights into the ideal approach to solving complex problems. By studying these samples, individuals can gain a better understanding of effective strategies, common errors, and the essential elements needed for a complete and well-structured answer. This process allows you to recognize how to organize thoughts and apply concepts efficiently.
While analyzing these examples, focus on several critical components to fully comprehend how to approach similar tasks in the future:
- Logical Structure: How well are the steps organized? Is the explanation clear and easy to follow?
- Accuracy: Are the calculations or theoretical concepts correct? Are all components, such as formulas and values, used appropriately?
- Thoroughness: Does the solution address all parts of the problem? Is there any missing information or detail that could have enhanced the response?
- Efficiency: Does the answer provide just enough detail, or does it include unnecessary information?
Example Breakdown
The following table illustrates a detailed breakdown of a sample solution, showing its strengths and areas for improvement:
| Area |
Strengths |
Weaknesses |
| Logical Structure |
The steps are presented in a clear, sequential manner, making it easy to follow. |
The explanation of one step could benefit from further elaboration to avoid confusion. |
| Accuracy |
All the formulas are correctly applied, and the final results are precise. |
There are no errors in the calculations or logic. |
| Thoroughness |
All aspects of the question are addressed comprehensively. |
No missing information or steps are noted. |
| Efficiency |
Lessons Learned from 2009 Responses
Reviewing past solutions provides invaluable insights into how to approach complex problems. By analyzing past examples, it is possible to identify common mistakes, successful strategies, and best practices for crafting well-rounded solutions. Reflecting on these lessons helps to improve performance and refine problem-solving techniques for future challenges.
The following key takeaways can significantly impact how one approaches similar tasks in the future:
- Attention to Detail: Small mistakes, such as miscalculating values or overlooking crucial steps, can lead to incorrect answers. Ensuring thoroughness and accuracy is essential.
- Clear Explanation: Well-structured and clearly articulated explanations are crucial for demonstrating reasoning. Avoid vague responses that leave the reader questioning the steps taken.
- Breaking Down Complex Tasks: Large problems can often feel overwhelming, but breaking them down into manageable parts makes them more approachable and easier to solve.
- Following Instructions Carefully: Always ensure that all aspects of the problem are addressed. Pay close attention to instructions and requirements to ensure completeness.
- Efficiency in Communication: While thoroughness is important, being concise and avoiding unnecessary information is equally vital. Keep explanations to the point and relevant to the question at hand.
By incorporating these lessons into future approaches, individuals can significantly improve their problem-solving abilities. Learning from past responses helps to avoid common pitfalls, making it easier to tackle challenging problems with confidence and clarity.
Improving Problem-Solving Skills
Developing strong problem-solving abilities is essential for tackling complex tasks and finding effective solutions. It requires not only knowledge of the subject but also the ability to think critically, approach challenges methodically, and apply strategies that lead to success. Enhancing these skills allows individuals to navigate difficult questions more efficiently and with greater confidence.
To improve problem-solving capabilities, consider the following approaches:
- Practice Regularly: Consistent practice is the foundation of improvement. Regularly solving a variety of problems helps strengthen understanding and build familiarity with different types of challenges.
- Understand the Process: Instead of focusing only on the final answer, concentrate on understanding the steps involved in reaching a solution. This deeper comprehension enhances the ability to handle more complicated questions.
- Work Backwards: When facing particularly difficult tasks, try working from the solution back to the starting point. This approach can provide insights into the steps required to solve the problem.
- Seek Different Perspectives: When encountering obstacles, seek alternative methods or explanations. Viewing the problem from multiple angles can reveal new strategies or simplify the approach.
- Analyze Mistakes: Review errors and identify where things went wrong. Understanding why a particular approach did not work helps to avoid repeating the same mistakes and improves future decision-making.
By consistently applying these methods and maintaining a focused approach, individuals can gradually sharpen their problem-solving skills, making them more adept at handling both simple and complex challenges.
Review of Thermodynamics Questions
Understanding thermodynamics is crucial for addressing questions that involve energy changes, heat transfer, and the behavior of systems. These questions often require knowledge of key principles such as the laws of thermodynamics, entropy, enthalpy, and Gibbs free energy. A strong grasp of these concepts allows for the ability to analyze systems and predict the direction of spontaneous processes.
Key Concepts to Focus On
When reviewing thermodynamics-related questions, it’s important to focus on the following concepts:
- First Law of Thermodynamics: This law, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transformed from one form to another.
- Entropy: Entropy measures the disorder or randomness of a system. Understanding how entropy changes during reactions is vital for predicting spontaneity.
- Enthalpy: The enthalpy change of a reaction indicates whether a process is endothermic or exothermic. Recognizing the relationship between heat and enthalpy is key for solving related problems.
- Gibbs Free Energy: This concept is essential for determining whether a reaction will occur spontaneously. A negative Gibbs free energy indicates spontaneity, while a positive value suggests non-spontaneity.
Common Strategies for Solving Thermodynamics Problems
When tackling thermodynamics problems, apply these strategies for better results:
- Identify Known and Unknown Quantities: Start by identifying what is given in the problem and what you need to find. This helps to narrow down the approach you will take.
- Apply the Relevant Equations: Use the appropriate formulas for enthalpy, entropy, and Gibbs free energy to calculate the unknowns. Ensure you understand when and how to apply each equation.
- Consider Directionality: Determine whether a reaction or process is spontaneous by considering the signs of entropy and Gibbs free energy changes.
- Check Units and Conversions: Thermodynamics calculations often require unit conversions. Double-check units to ensure consistency across all terms and to avoid calculation errors.
By mastering these concepts and applying the suggested strategies, you will be well-prepared for any thermodynamics-related question that comes your way.
Understanding Chemical Equilibria
Grasping the concept of equilibrium is essential for analyzing reactions that do not go to completion but instead reach a state of balance between the forward and reverse processes. In such systems, the concentrations of reactants and products remain constant over time, although the reactions continue to occur. A deep understanding of equilibrium helps predict the behavior of chemical systems under varying conditions.
Key Concepts in Equilibria
Several crucial ideas are involved in understanding equilibrium:
- Dynamic Nature: Although the concentrations of reactants and products remain constant, both the forward and reverse reactions continue at the same rate, making the system dynamic in nature.
- Le Chatelier’s Principle: This principle states that when a system at equilibrium is subjected to a change (in concentration, pressure, or temperature), the system will adjust to counteract the change and restore equilibrium.
- Equilibrium Constant (K): The equilibrium constant, K, expresses the ratio of product concentrations to reactant concentrations, each raised to the power of their respective coefficients in the balanced equation. It helps determine the position of equilibrium.
- Reaction Quotient (Q): Similar to the equilibrium constant, Q is used when a reaction is not yet at equilibrium. Comparing Q with K helps predict the direction of the reaction.
Solving Equilibrium Problems
To effectively solve problems related to equilibrium, follow these steps:
- Write the Balanced Equation: Ensure that the reaction is balanced, including the correct stoichiometric coefficients for both reactants and products.
- Set Up the Expression for K: Express the equilibrium constant based on the concentrations of products and reactants, and substitute the given values into the expression.
- Calculate Using ICE Tables: ICE (Initial, Change, Equilibrium) tables are useful for organizing information on concentrations and calculating the equilibrium concentrations of reactants and products.
- Apply Le Chatelier’s Principle: If the problem involves a change in concentration, pressure, or temperature, predict how the system will shift to restore equilibrium.
By mastering these concepts and strategies, you will be well-prepared to handle equilibrium-related questions and better understand the behavior of chemical reactions in dynamic systems.
Preparing for Future Exams
Preparing for upcoming exams requires a strategic approach that balances review, practice, and time management. The goal is to not only recall information but also to develop a deep understanding of key concepts. Consistent study, active engagement with materials, and targeted practice are essential for performing well under pressure.
Effective Study Techniques
To maximize study sessions, consider the following strategies:
- Active Recall: Instead of passively reading notes, actively test yourself on key concepts to strengthen memory retention.
- Spaced Repetition: Reviewing material at spaced intervals helps to improve long-term retention and reduces cramming.
- Practice Problems: Solving a variety of problems helps reinforce concepts and improve problem-solving skills, especially under exam conditions.
- Concept Mapping: Creating visual aids, such as concept maps, helps organize complex information and improves understanding.
Time Management Strategies
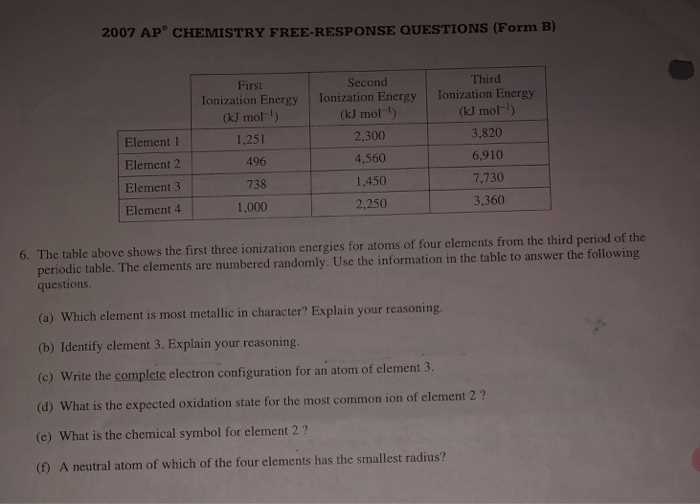
Proper planning is essential to ensure adequate coverage of all topics. Here are some tips:
| Strategy |
Benefits |
| Study Plan |
Organizes topics by priority and allocates study time effectively. |
| Pomodoro Technique |
Helps maintain focus by alternating between focused study sessions and short breaks. |
| Daily Reviews |
Reinforces material learned earlier and prevents last-minute cramming. |
By integrating these study techniques and time management practices, you can enhance your preparation and boost your confidence as the exam day approaches. Consistency, planning, and active engagement are the keys to success.
Resources for Further Practice
To excel in any exam, consistent practice is crucial. Accessing high-quality resources can help reinforce understanding, develop problem-solving skills, and boost confidence. A combination of textbooks, online platforms, and interactive tools can provide valuable support in your preparation process.
Textbooks and Study Guides
Textbooks offer in-depth explanations of essential concepts and provide a strong foundation. Some additional study guides and reference materials include:
- Textbook Solutions: Review example problems and their step-by-step solutions to reinforce key principles.
- Review Guides: Comprehensive summaries of important topics, often accompanied by practice questions and solutions.
- Study Workbooks: Designed for practice, these workbooks often contain exercises for self-assessment and review.
Online Learning Platforms
Online platforms offer interactive resources that cater to various learning styles. These can be especially helpful for honing specific skills or reviewing difficult concepts:
- Interactive Quizzes: Many websites provide quizzes and tests that simulate real exam conditions, helping you track progress.
- Video Lessons: Visual and auditory learners can benefit from video tutorials that break down complex ideas into understandable segments.
- Practice Exams: Full-length practice exams allow you to experience the exam format and timing, which is vital for building test-taking strategies.
Mobile Apps and Flashcards
Using mobile apps and flashcards is an excellent way to study on the go. They allow you to review concepts in short bursts, which can be more effective than long study sessions:
- Flashcard Apps: Apps like Quizlet allow you to create custom flashcards or use pre-made sets to test your knowledge.
- Mobile Study Apps: Many apps focus on subject-specific topics and allow you to practice anywhere, anytime.
Incorporating these resources into your study routine can significantly enhance your preparedness, allowing you to tackle challenges with confidence and clarity.
Insights from Past AP Students
Learning from the experiences of those who have previously faced the same challenges can provide valuable insights into effective strategies for success. Many former students share their advice, offering tips on how to approach the material, manage time, and navigate the pressures of rigorous assessments. By understanding what worked for them, you can adapt their techniques to enhance your own preparation.
Time Management and Prioritization
One common theme among past students is the importance of managing time effectively. Many recommend setting aside dedicated time each day for study, while also balancing rest and other commitments. Here are some key strategies:
- Create a Study Schedule: Organize your study sessions by topic, allowing sufficient time for each area based on its complexity.
- Break Down Tasks: Avoid overwhelming yourself by breaking large tasks into smaller, manageable parts.
- Prioritize Weak Areas: Focus on areas where you need the most improvement, but be sure to review strengths as well.
Active Learning and Practice
Another piece of advice often shared by previous students is to focus on active learning rather than passive reading. Engaging directly with the material helps deepen understanding. Some effective techniques include:
- Practice Problems: Regularly solving practice questions reinforces learning and helps identify areas where further study is needed.
- Group Study: Collaborating with peers can provide different perspectives and can help reinforce concepts that might be difficult to grasp on your own.
- Simulate Test Conditions: Taking practice exams under timed conditions helps improve time management and builds confidence for the actual test.
By adopting these strategies and insights from past students, you can create a personalized approach to your studies, better preparing yourself for success.