
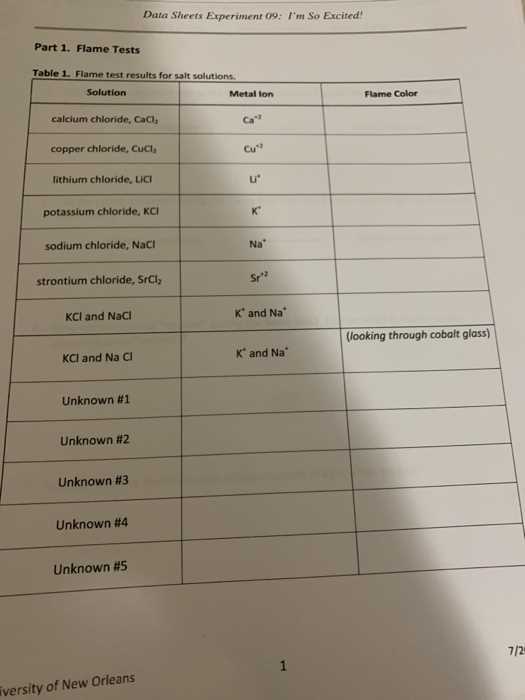
In scientific experiments, the color produced when certain materials are exposed to heat can reveal important details about their composition. This method has been used for years as a simple yet effective means of identifying specific substances based on their unique reactions to high temperatures. Each element emits a distinct color, making this technique a valuable tool for chemists and researchers alike.
During these experiments, a small sample of a substance is heated to the point where its electrons become excited. As they return to their normal state, they release energy in the form of light, which is visible as color. The shade of this light depends on the energy levels of the element’s electrons, allowing for precise identification when compared to known standards.
This section will explore how various elements are recognized through their characteristic responses to heat and provide insights into the key principles that govern these reactions. Whether you’re preparing for a study session or aiming to understand the scientific reasoning behind these colorful phenomena, this guide will cover the fundamentals and beyond.
Flame Lab Test Answers
In scientific experimentation, the heat-induced reactions of materials provide valuable insights into their composition. These reactions produce distinct colors when certain elements are exposed to high temperatures. By observing and analyzing these color changes, researchers can identify the substances present in a sample. This process has become a standard method for detecting various chemical elements in both educational and professional settings.
Key Elements and Their Reactions
Each element has a unique response when subjected to heat, releasing light at specific wavelengths. For example, lithium gives off a bright red hue, while copper emits a greenish-blue glow. Understanding these color signatures is crucial in identifying substances accurately. The color variations are the result of the interaction between the element’s electrons and the energy from the heat, which allows for identification based on these distinct emissions.
Common Issues and Troubleshooting
While this method is generally reliable, several factors can influence the results. Impurities in the sample, incorrect heating, or contamination can lead to misleading colors. Therefore, maintaining the proper technique and following strict guidelines is essential for achieving accurate results. Troubleshooting common issues, such as inconsistent or faint colors, often involves adjusting the temperature, ensuring clean equipment, and verifying the sample’s purity.
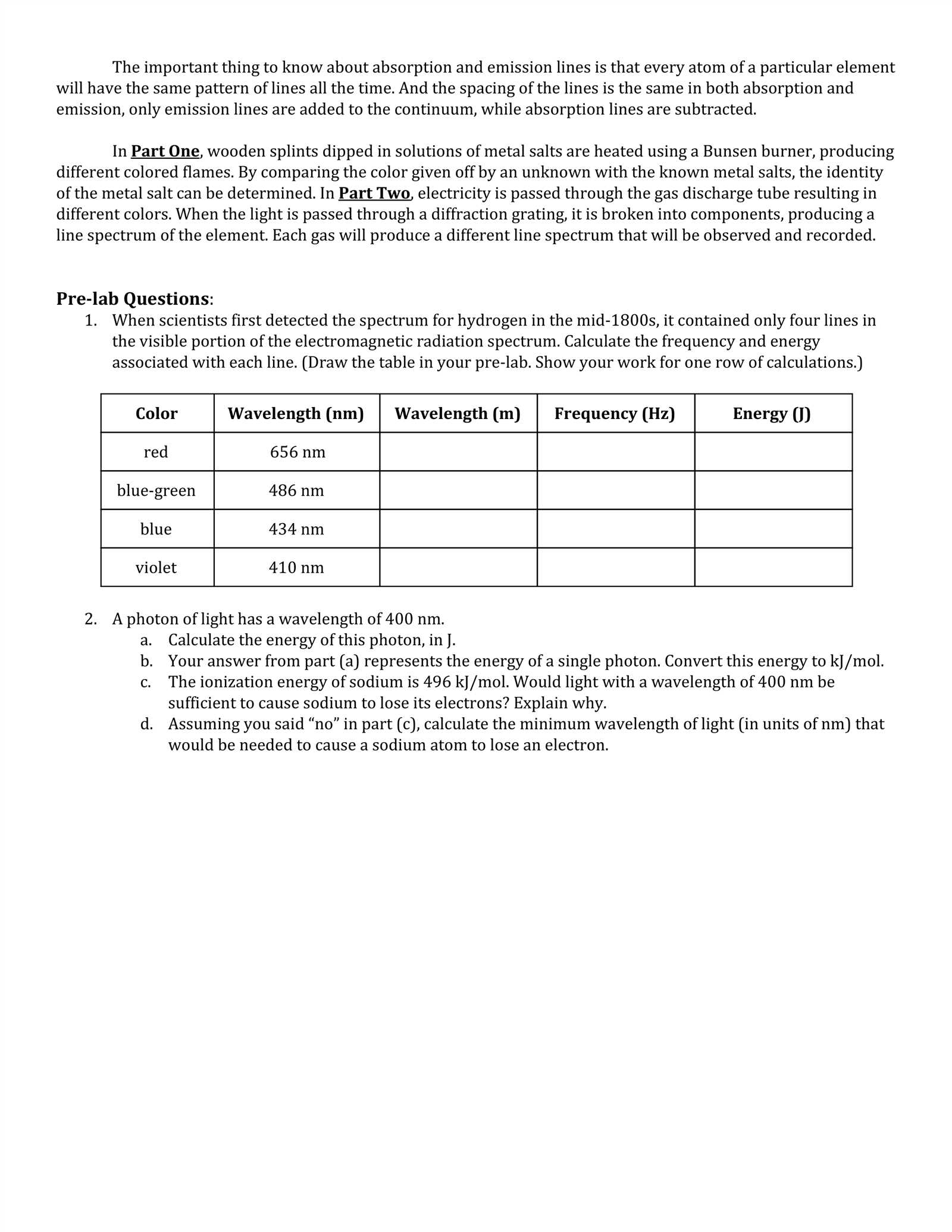
Understanding Heat-Induced Color Reactions in Chemistry

In chemistry, certain substances reveal their composition through a distinctive color when exposed to intense heat. This reaction occurs as the electrons within an element become excited and release energy in the form of visible light. The emitted light is characteristic of the element, allowing chemists to identify the substance based on its specific color signature. This method provides a quick and effective way to analyze the chemical makeup of unknown materials.
The Science Behind the Color Emission
When a chemical element is heated, its electrons absorb energy and jump to higher energy levels. As they return to their original state, they release the absorbed energy in the form of light. The wavelength of this light corresponds to the difference in energy levels between the excited and ground states of the electrons. Each element has a unique electron configuration, resulting in distinct color emissions that can be observed and used for identification.
Common Elements and Their Color Reactions
Different elements produce unique colors when heated. The following table summarizes the color reactions of several common elements:
| Element | Color Emission |
|---|---|
| Lithium | Crimson Red |
| Sodium | Bright Yellow |
| Potassium | Light Violet |
| Calcium | Orange-Red |
| Copper | Greenish-Blue |
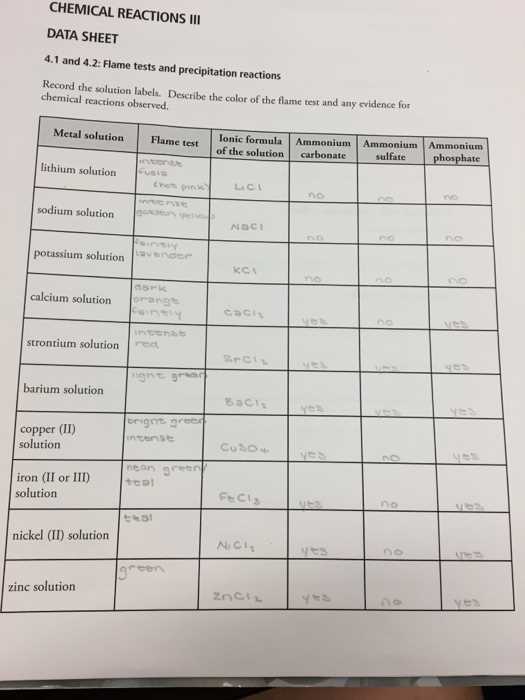
Common Elements in Heat-Induced Color Reactions
Various elements exhibit distinct color emissions when subjected to intense heat. These reactions are used to identify the presence of specific substances by observing the light released during the heating process. Each element’s electron configuration determines the unique wavelengths of light it emits, providing a reliable way to detect and analyze materials in both educational and professional settings.
Key Elements and Their Reactions
Some elements are more commonly tested due to their distinctive and easily recognizable color reactions. The following table highlights several elements and the colors they produce when heated:
| Element | Color Emission |
|---|---|
| Lithium | Crimson Red |
| Sodium | Bright Yellow |
| Potassium | Light Violet |
| Calcium | Orange-Red |
| Barium | Green |
Understanding Variations in Color
The variations in color produced by these elements are due to the differences in their atomic structures. The energy levels in atoms determine how much energy is needed to excite electrons and the wavelength of light they emit as they return to their original state. These distinct color signatures can be used to distinguish elements in various chemical analyses.
Role of Heat-Induced Colors in Identification
The colors produced when certain substances are exposed to heat play a crucial role in identifying their chemical composition. Each element releases a unique color when heated, allowing scientists to distinguish between different substances based on their light emission. This characteristic is used to determine the presence of specific elements, making it an invaluable tool in both laboratory and field analyses.
How Colors Aid in Identification
When a substance is heated, its electrons become excited and jump to higher energy levels. As they return to their lower energy states, they release energy in the form of visible light. The wavelength of this emitted light is unique to each element, providing a visual signature that can be matched to known standards. By comparing the observed color with a reference chart, chemists can accurately identify the element present in the sample.
Practical Applications of Color Reactions
These heat-induced color reactions are widely used in various fields, including educational experiments, forensic investigations, and material testing. They provide a simple yet effective method of element identification, allowing researchers to quickly gather information without the need for more complex and time-consuming procedures. Understanding the role of these color changes is essential for interpreting results and ensuring accurate identification of substances.
Preparing for a Heat-Induced Reaction Experiment
Proper preparation is key to successfully conducting an experiment that involves heat reactions to identify chemical substances. Understanding the steps involved, gathering the right materials, and ensuring safety are all critical aspects of the process. Careful planning ensures that the experiment runs smoothly and that results are both accurate and reliable.
Essential Materials and Equipment
Before starting, make sure to gather all necessary materials. This includes the substances to be tested, a heat source, and tools to handle the samples. The following table outlines the common equipment needed for such experiments:
| Equipment | Purpose |
|---|---|
| Bunsen Burner | Provides controlled heat to excite the elements |
| Sample Holder | Holds the substance being tested |
| Wire Loop | Used to dip into the sample for heating |
| Safety Goggles | Protects eyes from potential hazards |
| Heat-Resistant Gloves | Protects hands while handling hot equipment |
Understanding Safety Protocols
Safety is the top priority when performing any experiment involving heat. Always wear appropriate protective gear such as goggles and gloves. Ensure that the workspace is well-ventilated and free from flammable materials. Additionally, it is essential to have a fire extinguisher or safety shower nearby in case of emergencies. Following established safety protocols will help prevent accidents and ensure a safe working environment.
Key Factors Affecting Heat-Induced Color Emissions
The color produced when a substance is heated can vary depending on several factors. These variations are influenced by the nature of the element, the conditions under which it is heated, and the composition of the sample. Understanding these key factors is essential for accurate interpretation of the color produced during the heating process.
One of the most significant factors is the element’s atomic structure. Each element has a unique arrangement of electrons, and when they are excited by heat, the energy released as they return to their normal state results in a specific color. The intensity of the heat also plays a crucial role; higher temperatures can cause a broader range of colors to emerge. Additionally, the presence of impurities in the sample can alter the emission, often leading to unexpected color changes.
Environmental conditions such as the presence of oxygen or other gases can also impact the emitted color. For instance, certain elements may emit different hues in a low-oxygen environment compared to when they are exposed to air. Understanding these variables helps in achieving more precise and consistent results when observing heat-induced reactions.
Interpreting Results in Heat-Triggered Reactions
After conducting a heat-triggered reaction, it is important to accurately interpret the resulting colors in order to identify the chemical composition of the sample. The observed color changes provide valuable insights into the elements present, but careful analysis is necessary to ensure correct conclusions are drawn.
To properly interpret the results, follow these key steps:
- Compare Observed Colors with a Reference Chart: Each element produces a unique color when heated. Referencing a color chart specific to the substances being tested is essential for accurate identification.
- Consider the Intensity of the Color: The intensity or brightness of the color can indicate the concentration of the element in the sample. Stronger colors often suggest a higher concentration, while faint hues may point to trace amounts.
- Account for Environmental Factors: Factors like temperature variations, the presence of impurities, and oxygen levels can influence the emitted colors. Make sure to control or document these variables to avoid misinterpretation.
- Cross-Check Results with Other Methods: While color changes are a reliable indicator, cross-checking results with additional methods such as spectroscopy or chemical reactions can provide further validation.
By following these guidelines, you can ensure that the results of your heat-triggered reactions are interpreted accurately and lead to meaningful conclusions about the sample’s composition.
Common Mistakes in Heat-Triggered Reactions
When performing experiments that involve heating substances to observe color changes, it is easy to make mistakes that can lead to inaccurate results or misinterpretations. These errors often arise from a lack of attention to detail, improper procedures, or external factors that influence the reaction. Understanding common pitfalls can help ensure more reliable and consistent outcomes.
Frequent Errors in Procedure
- Incorrect Sample Handling: Using improper tools or touching the sample directly can lead to contamination or altered results. Always use clean instruments and avoid handling substances with bare hands.
- Failure to Control Temperature: Inconsistent or inadequate heating can result in weak or inconsistent color emissions, making it difficult to identify the substance accurately. Ensure the heat source is at the appropriate temperature for the reaction.
- Overheating: Overheating the sample can cause degradation of the substance or lead to unexpected reactions that produce inaccurate colors. Avoid exposing the material to excessive heat for too long.
- Not Using a Reference Chart: Failing to compare the observed color with a reference chart can result in misidentification of the element. Always use a reliable color reference to ensure proper identification.
Environmental and External Factors
- Ignoring Ambient Conditions: External factors like humidity, air quality, and oxygen levels can impact the color emission. Always be aware of the conditions in the environment that could affect the results.
- Contamination from Impurities: Impurities in the sample or in the heating equipment can cause unexpected color reactions, leading to confusion. Ensure samples are pure and equipment is thoroughly cleaned before use.
Avoiding these common mistakes ensures more reliable results and helps in accurately identifying substances based on their heat-induced color reactions.
Best Practices for Heat-Induced Reaction Accuracy
Ensuring accuracy in experiments that involve heating substances for identification is essential for obtaining reliable results. Following best practices not only improves the precision of the observations but also enhances the overall efficiency of the procedure. Careful attention to detail, proper technique, and controlling environmental factors can significantly reduce errors and increase the reliability of the outcomes.
Essential Techniques for Reliable Results
- Use High-Quality Reference Materials: Always use a reliable color reference chart when interpreting the results. Having access to a well-maintained chart will help you compare the emitted colors with known standards.
- Calibrate Equipment Regularly: Make sure that all heating devices are properly calibrated and functioning. Inaccurate heating can result in unpredictable outcomes and affect the accuracy of your results.
- Use Proper Sample Preparation: Ensure that the sample is clean, well-prepared, and free from contaminants that could skew the results. Handling materials with clean, sterile tools helps avoid unwanted reactions.
- Maintain Consistent Heat Application: Apply heat steadily and evenly. Fluctuating temperatures can cause inconsistent reactions and make it difficult to interpret the results accurately.
Control External Variables
- Monitor Environmental Conditions: Pay attention to factors such as ambient temperature, humidity, and air composition. These variables can influence the reaction and lead to inconsistent results.
- Limit External Contamination: Prevent contamination by using clean tools and working in a controlled environment. Even small impurities can significantly alter the outcome.
- Use Correct Sample Quantities: Avoid using too much or too little of the sample. Too much material can affect the intensity of the color emitted, while too little may produce faint or undetectable results.
By adhering to these best practices, you can significantly enhance the reliability and accuracy of your heat-induced reactions, ensuring that results are consistent and meaningful for further analysis.
Understanding the Chemistry Behind Heat-Induced Colors

The colors produced when substances are heated are a direct result of atomic interactions and energy transitions. When atoms absorb energy, their electrons move to higher energy levels. As the electrons return to their original or lower energy states, they release energy in the form of light, which manifests as color. The specific wavelength of light emitted depends on the element’s unique atomic structure.
To understand these color emissions in more depth, it’s crucial to consider the following factors:
The Role of Electrons
- Electron Excitation: When an atom absorbs heat, its electrons gain energy and move to higher energy levels. This is called excitation.
- Electron Relaxation: After excitation, electrons return to their lower energy states, releasing energy in the form of visible light. The color observed depends on the amount of energy released during this process.
Key Factors Affecting Emission
- Element’s Atomic Structure: Each element has a distinct electron configuration, which determines the specific wavelengths of light it emits. This results in characteristic colors for different elements.
- Energy Level Transitions: The greater the difference between energy levels, the higher the energy and shorter the wavelength of light emitted, leading to a more intense color.
- Temperature: The amount of heat applied influences the extent of electron excitation. Higher temperatures may cause a broader spectrum of colors to appear, as more electrons are excited to higher levels.
By understanding these fundamental chemical principles, we can better interpret the color changes observed in various heat-based reactions and identify the substances involved.
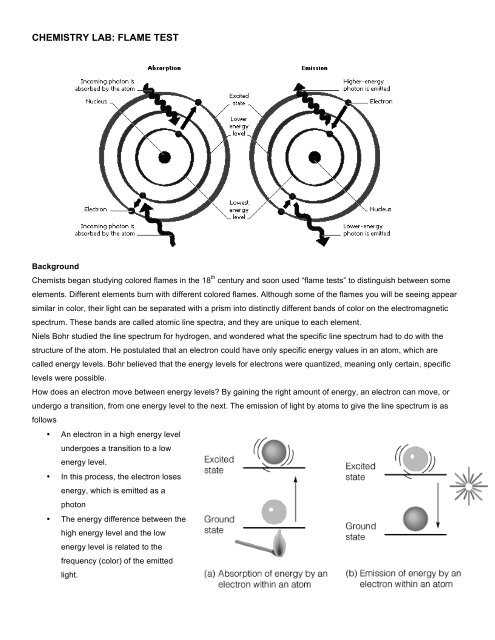
Identifying Elements Through Heat-Induced Color Changes
The process of identifying elements through heat-induced color changes relies on the unique light emissions produced by each element when heated. When certain substances are exposed to heat, their electrons absorb energy and transition to higher energy levels. As the electrons return to their original states, they release energy in the form of light, which can be observed as specific colors. These colors are unique to each element and can be used to identify the presence of particular substances in a sample.
Common Elements and Their Colors

The colors emitted during heating are influenced by the atomic structure of the element. Each element has a characteristic emission spectrum, which produces a distinct color. Below is a table showing some common elements and their corresponding colors:
| Element | Color Emitted |
|---|---|
| Sodium | Bright Yellow |
| Potassium | Light Lilac |
| Calcium | Orange-Red |
| Copper | Green |
| Strontium | Crimson Red |
| Barium | Greenish Yellow |
Procedure for Identifying Elements
- Prepare the Sample: A small amount of the substance is placed on a non-reactive wire or support.
- Apply Heat: The sample is heated using a Bunsen burner or another appropriate heat source until the desired color appears.
- Observe the Color: The emitted color is carefully observed and compared with known standards to identify the element.
- Record the Results: The color is documented for further analysis and identification.
This technique provides a quick and effective way to identify elements, especially in educational or laboratory settings, and is valuable for confirming the composition of unknown substances.
Heat-Induced Reaction Safety Guidelines

When performing experiments involving heating substances to observe color changes, safety is a top priority. The process can involve open flames and potentially hazardous chemicals, so it’s essential to follow proper safety procedures to prevent accidents and ensure a safe environment. By adhering to safety guidelines, individuals can minimize risks and handle materials responsibly during these activities.
General Safety Precautions

- Wear Protective Gear: Always wear safety goggles, gloves, and a lab coat to protect yourself from harmful chemicals and heat exposure.
- Ensure Proper Ventilation: Conduct the experiment in a well-ventilated area, such as a fume hood, to avoid inhaling any fumes or gases that may be released during the heating process.
- Check Equipment: Before beginning, ensure that all equipment, such as heat sources and containers, are in good working condition to prevent accidents.
Handling Chemicals and Heat Sources
- Properly Store Chemicals: Store chemical reagents in labeled, secure containers and keep them away from open flames or excessive heat sources.
- Monitor Heat Levels: Never leave heating sources unattended. Always monitor the process closely to prevent overheating or unsafe reactions.
- Dispose of Materials Safely: After completing the experiment, dispose of any chemical waste according to your institution’s guidelines to prevent contamination or chemical reactions.
By following these guidelines, you can ensure a safer and more controlled environment when performing heating-based experiments, minimizing the risk of injury and maximizing the reliability of results.
Using Heat-Based Methods for Qualitative Analysis
Heat-induced color reactions are commonly employed in qualitative analysis to identify the presence of specific elements in a sample. This technique relies on the unique light emission characteristics of elements when they are heated. Each element emits a distinct color as its atoms or ions undergo energy transitions, making it a useful tool for identifying substances based on their emission spectra. Through careful observation and comparison with known standards, chemists can confirm the composition of unknown samples.
In qualitative analysis, this method serves as a simple yet effective approach to determine the elemental makeup of a substance. While it is not suitable for quantifying the amount of an element, it provides immediate, observable results that help confirm the identity of a material. By examining the emitted colors, analysts can identify a variety of metals and salts, making it an essential technique in fields ranging from education to industrial applications.
Limitations of Heat-Induced Color Reaction Methodology
While heat-based color reactions are a valuable tool for identifying certain elements, they come with several limitations that can impact the accuracy and reliability of the results. One primary constraint is the interference from complex mixtures. When a sample contains multiple elements, their emission spectra may overlap, making it difficult to distinguish between them accurately. This limits the technique’s ability to identify elements in highly mixed or impure substances.
Another limitation is the sensitivity of the method. Some elements may require very high temperatures to emit detectable colors, while others may produce faint or indistinguishable emissions. As a result, elements that do not produce strong colors or are present in trace amounts may go undetected. Additionally, the technique is not effective for determining the quantity of an element in a sample; it is solely qualitative. These factors must be considered when deciding whether to rely on this methodology for identification purposes.
Heat-Based Reactions vs Other Analytical Methods
Heat-induced color reactions are a straightforward and quick method for identifying certain elements in a sample. However, compared to other analytical techniques, they are often less precise and more limited in their application. Unlike instrumental methods such as atomic absorption spectroscopy (AAS) or inductively coupled plasma (ICP) analysis, heat-induced color reactions do not provide detailed quantitative information or the ability to analyze complex mixtures with high accuracy. They are primarily used for rapid, qualitative analysis rather than in-depth compositional analysis.
While heat-based reactions are highly effective for detecting specific elements based on their characteristic colors, they have drawbacks in terms of sensitivity and selectivity. In contrast, techniques like AAS or ICP can measure the concentration of elements with much greater precision and are capable of handling a broader range of sample types. These methods also allow for the detection of trace amounts, which heat-based reactions may miss. Therefore, while heat-induced color reactions are valuable for simple, initial screenings, other analytical methods are preferred for more comprehensive, accurate, and quantifiable results.
Advanced Techniques in Heat-Induced Emission Analysis
While traditional heat-induced emission methods are useful for basic element identification, recent advancements in analytical technology have enhanced the sensitivity, precision, and range of these techniques. One such development is the use of flame atomic emission spectroscopy (FAES), which allows for more precise measurement of the intensity of light emitted by atoms in a heated state. This method significantly improves the detection limits and is capable of analyzing a wider variety of samples with greater accuracy compared to basic color-based reactions.
Another innovative technique is the inductively coupled plasma optical emission spectrometry (ICP-OES), which combines the power of high-temperature plasma with advanced optical emission detection. This method not only increases sensitivity but also expands the ability to identify a broader range of elements simultaneously. With ICP-OES, the results are highly reproducible and allow for detailed quantitative analysis, offering a comprehensive approach to sample analysis.
These advanced methods overcome the limitations of basic heat-based reactions, providing greater accuracy, precision, and the ability to handle complex samples. As a result, they are becoming more widely adopted in industries requiring detailed elemental analysis, such as environmental monitoring, pharmaceuticals, and material science.
Heat-Induced Emission Applications in Industry
The use of heat-induced emission methods has found diverse applications across various industries due to its simplicity, efficiency, and ability to provide quick results. These techniques are particularly valuable in fields where rapid identification and analysis of elemental composition are essential. The ability to detect specific metals and compounds makes these methods indispensable in quality control, material testing, and environmental monitoring.
Applications in Manufacturing and Quality Control
In manufacturing, especially in the production of metals and alloys, heat-induced emission analysis is widely employed for quality assurance. The technique helps ensure that raw materials meet the required specifications, and it is also used for monitoring the consistency of production processes.
- Metals and Alloys Production: To check the purity and composition of metals such as copper, aluminum, and steel.
- Quality Control: In industries like automotive and aerospace, to verify material properties and prevent defects in components.
- Plastic Manufacturing: Identifying trace elements in resins and polymers to ensure product quality.
Environmental and Waste Management
In environmental monitoring, these techniques are crucial for analyzing water, soil, and air samples. The ability to identify harmful contaminants quickly and accurately supports efforts in pollution control and regulatory compliance.
- Water Quality Monitoring: To detect heavy metals and other pollutants in drinking and wastewater.
- Soil Analysis: Identifying metal contamination in agricultural fields or industrial sites.
- Air Quality Control: Monitoring emissions from industrial plants to comply with environmental regulations.
Overall, heat-induced emission analysis continues to play a significant role in industrial applications, offering a rapid and cost-effective way to analyze and maintain the quality of materials while ensuring safety and compliance with environmental standards.
Troubleshooting Common Issues in Heat-Induced Emission Analysis
Despite the simplicity and effectiveness of heat-induced emission methods, several issues can arise during the process that may affect the accuracy and reliability of the results. Identifying and addressing these challenges promptly ensures more accurate outcomes and a smoother analysis procedure. The following outlines some common problems and their solutions, helping to optimize the analytical process.
1. Inconsistent or Faint Emission Colors
One of the most common issues faced during this process is inconsistent or faint color emissions, which can lead to difficulty in identifying the elements present in a sample. This problem may stem from several factors, including improper sample preparation or incorrect flame temperature.
- Solution: Ensure the sample is clean and free of contaminants that might interfere with the process. Additionally, verify that the heat source is functioning properly and that the correct temperature range is achieved to excite the elements.
- Solution: Adjust the distance between the sample and the heat source, as being too far away can result in insufficient emission.
2. Overlapping Emissions

When multiple elements are present in a sample, their emissions might overlap, making it difficult to distinguish between them. This issue can be especially troublesome when working with complex mixtures.
- Solution: Use a more refined method to isolate individual elements, such as reducing the concentration of the sample or performing a separation step before analysis.
- Solution: Consider using specialized filters or spectrometers that can differentiate between closely spaced wavelengths, providing clearer results.
3. Contamination of Equipment
Equipment contamination is another common problem that can affect the quality of the results. Residue from previous samples, or impurities in the equipment, can alter emission readings and lead to incorrect conclusions.
- Solution: Regularly clean all equipment, including the heat source and sample holders, using appropriate cleaning agents that do not introduce contaminants.
- Solution: Replace or maintain equipment components, such as burner tips or nozzles, that may degrade over time due to wear and buildup.
By understanding and addressing these common issues, analysts can significantly improve the reliability of heat-induced emission methods, ensuring more precise and consistent results for all types of samples.