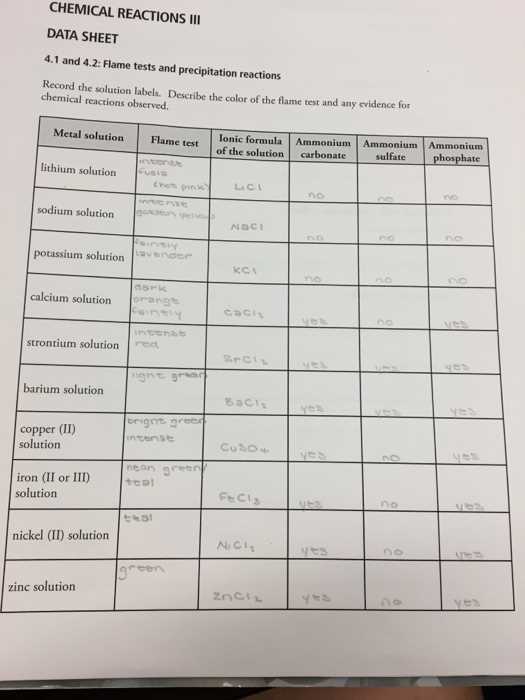
In scientific experiments, the ability to identify elements based on their behavior in certain conditions is essential. One such method involves observing how substances react when exposed to heat, revealing distinct visual signatures. This technique plays a crucial role in helping students and professionals understand the properties of various elements and compounds.
Before engaging in hands-on experimentation, it’s important to have a clear understanding of the procedures and expected outcomes. Gaining insights into the reactions and learning how to correctly interpret the results can significantly enhance the overall experience. This preparation not only increases accuracy but also builds a deeper understanding of the science behind the reactions.
Through this guide, individuals can familiarize themselves with the necessary steps, common outcomes, and key concepts to ensure success during practical applications. Mastering these concepts is fundamental for anyone looking to deepen their knowledge of elemental analysis and chemical behavior.
Flame Test Pre Lab Answer Key

Before conducting any experiment involving the observation of elemental reactions to heat, it is crucial to have a solid understanding of the process. Preparing for such experiments involves familiarizing oneself with the typical outcomes and the steps needed to carry out the procedure correctly. This knowledge serves as a foundation for successful experimentation and helps in accurately interpreting the results.
Preparing for the Experiment
Proper preparation is key to ensuring the experiment goes smoothly. Understanding the various reactions that can occur when different substances are exposed to high temperatures will help students recognize the patterns in their observations. Each element produces a specific color when subjected to heat, and this color serves as an indicator of its identity. Knowing what to expect allows participants to be more focused and accurate in their analysis.
Common Reactions and Observations

In most cases, the behavior of metals and compounds when heated results in vivid color changes. For instance, elements such as sodium, copper, and potassium each display unique hues, which are essential for their identification. These color shifts are due to the energy emitted when electrons within the atoms transition between different energy levels. A clear understanding of these reactions is necessary to draw the correct conclusions about the elements being tested.
Understanding Flame Test Basics

In scientific exploration, certain elements and compounds exhibit unique visual characteristics when exposed to heat. These characteristics are crucial for identifying substances, making this method a valuable tool in both educational and research settings. The phenomenon occurs when electrons within an atom absorb energy and move to higher energy levels, subsequently releasing energy as light when they return to their original state. This process is the foundation for understanding how the procedure works.
The Role of Heat in Chemical Reactions
Heat plays a significant role in exciting the atoms or molecules within a substance. As the material reaches a high temperature, its electrons become energized and move to a higher orbit. When these electrons return to their lower energy states, they release energy in the form of light. The color of this emitted light corresponds to the energy difference between the electron orbits, which varies between elements. This distinct color serves as a crucial identifier for different elements and their compounds.
Identifying Elements by Color
Each element produces a specific color when subjected to heat. For example, copper can produce a greenish-blue hue, while lithium may produce a red flame. The colors are a direct result of the way the element’s electrons interact with the heat. Understanding which colors correspond to which substances allows scientists to quickly identify the composition of a material. This ability is fundamental in qualitative analysis and is widely used in both classrooms and professional labs.
Significance of Flame Test in Chemistry

This experimental method plays a crucial role in understanding the behavior of elements and their compounds. It provides valuable insights into the atomic structure of materials by allowing scientists to observe how they respond to heat. The visual outcome, often in the form of distinct color changes, reveals key information about the identity of substances, making it an essential technique for qualitative analysis.
The ability to identify specific elements based on their unique light emissions is a foundational concept in chemistry. This method is widely used in both educational settings and professional laboratories, helping to deepen understanding of atomic theory and electron behavior. By linking observable phenomena with theoretical concepts, this approach bridges the gap between practical experiments and scientific theory.
Additionally, this technique is highly effective in testing unknown substances, offering a simple yet reliable way to determine their chemical composition. It’s an indispensable tool for students, researchers, and professionals who need to quickly and accurately analyze the presence of various metals and compounds.
Common Elements Tested in Flame Tests
Certain metals and compounds are frequently analyzed using this heat-based method due to their unique responses when exposed to high temperatures. The specific colors emitted by these substances help identify their presence and provide important clues about their chemical composition. Below are some of the most commonly tested elements and the characteristic colors they produce during the experiment:
- Sodium (Na): Bright yellow flame
- Potassium (K): Light lilac or pale violet flame
- Copper (Cu): Green or blue-green flame
- Calcium (Ca): Orange-red flame
- Strontium (Sr): Red flame
- Ba (Barium): Green flame
- Litium (Li): Crimson red flame
Each of these elements exhibits a specific color due to the way their electrons absorb and release energy when exposed to heat. Understanding the unique color signatures of these materials is key for identifying them in various chemical experiments.
How to Prepare for a Flame Test
Preparation for this experiment is essential to ensure accurate results and a safe working environment. It involves gathering the appropriate materials, understanding the procedure, and following safety guidelines to avoid accidents. Proper preparation helps students and researchers gain the most from the experiment and increases the likelihood of successfully identifying the substances being studied.
Start by reviewing the elements or compounds you plan to analyze, and familiarize yourself with their expected reactions to heat. Ensure that you have all the necessary equipment, such as a heat source, wire loops, safety goggles, and appropriate chemicals. Before beginning the procedure, it’s important to check that all safety equipment is in good condition, and the area is well-ventilated to handle any potential fumes.
Once the materials and equipment are set up, take the time to review the steps involved. Knowing what to expect during the experiment will help you focus on the key observations, such as the color emitted by the substances when heated. Proper preparation also involves understanding the common errors that can arise, such as contamination of the sample, which could lead to misleading results.
Key Chemical Reactions in Flame Tests
The reactions observed during this procedure are a result of the interaction between heat and the electrons of elements and compounds. When heated, atoms absorb energy, causing electrons to move to higher energy levels. As these electrons return to their original states, they release energy in the form of light, which is what we observe as color. Each element has a unique emission spectrum, which makes this method a powerful tool for identifying substances based on their specific light emissions.
Electron Excitation and Emission
The core reaction that occurs during the experiment involves electron excitation. When an element is heated, its electrons gain enough energy to jump to a higher orbit. This state is unstable, so the electrons soon fall back to their lower energy levels, emitting light as they do. The color of this light corresponds to the energy difference between the orbits, which is unique for each element. This process is responsible for the distinct colors we observe in the reaction.
Influence of Metal Ions
The presence of metal ions significantly affects the color produced during the experiment. Each metal ion has a specific energy level and, therefore, a distinct wavelength of light that it emits. For example, sodium ions produce a bright yellow color, while copper ions can produce a greenish-blue hue. Understanding the role of metal ions and their characteristic emissions helps scientists identify elements with a high degree of accuracy during the analysis.
Identifying Colors in Flame Reactions
The colors emitted during a heating process provide crucial information about the composition of a substance. When certain materials are exposed to high temperatures, the energy excites the atoms within, causing them to release light at specific wavelengths. These wavelengths correspond to distinct colors, which can be used to identify the elements or compounds present in the sample. Understanding the significance of these colors is essential for accurately interpreting the results of the experiment.
Each element has a unique emission spectrum, which is essentially the pattern of colors it produces when heated. The energy released during electron transitions results in visible light of varying colors. For example, sodium typically emits a bright yellow hue, while potassium gives off a pale violet light. Identifying these colors allows scientists to match the observed reaction with the corresponding element, enabling accurate identification in a range of chemical analyses.
Interpreting Flame Test Results
Understanding the outcomes of heat-based reactions is crucial for correctly identifying the substances being studied. The colors produced during the experiment provide valuable insights into the presence of specific elements or compounds. However, accurate interpretation requires a solid understanding of how different materials react to heat and the factors that influence their emitted light. Interpreting these results allows for a clear connection between the observed color and the chemical composition of the sample.
Matching Colors to Elements

The color emitted by a substance when heated corresponds to the energy released as electrons return to their lower energy states. Each element has a characteristic color associated with this energy release, which can be compared to known references. For example, a bright yellow hue typically indicates the presence of sodium, while a greenish-blue light is often associated with copper. Recognizing these patterns is essential for accurate analysis in identifying elements.
Factors Influencing Color Interpretation
Several factors can affect the color observed during the experiment, including the temperature of the heat source, the purity of the sample, and the presence of impurities. Impurities or the presence of multiple elements can cause mixed or unexpected colors, making it important to carefully control experimental conditions. Additionally, the intensity and duration of the heat applied can influence the visibility of the color, so consistent methods are key to obtaining reliable results.
Safety Precautions During Flame Tests

Working with heat sources and chemicals requires careful attention to safety. When conducting experiments involving high temperatures, there are several precautions that must be taken to prevent accidents and ensure the well-being of everyone involved. Adhering to proper safety protocols helps mitigate risks such as burns, inhalation of toxic fumes, or accidental exposure to harmful substances.
Before beginning any experiment, it is important to ensure that the workspace is safe and all participants are aware of the necessary precautions. Below are some key safety measures to follow when handling materials and heat sources:
- Wear Proper Protective Gear: Always wear safety goggles, a lab coat, and heat-resistant gloves to protect yourself from potential burns or splashes.
- Work in a Well-Ventilated Area: Ensure that the workspace is properly ventilated to avoid inhaling any potentially harmful fumes or vapors released during the reaction.
- Use Proper Heat Sources: Use only approved heat sources such as a Bunsen burner or alcohol lamp, and always keep the flame at a safe distance from flammable materials.
- Avoid Contamination: Ensure that the materials being heated are free from impurities that could affect the experiment or lead to dangerous reactions.
- Know Emergency Procedures: Familiarize yourself with the location and proper use of fire extinguishers, safety showers, and eyewash stations.
- Handle Chemicals Carefully: Always follow the safety instructions for each chemical being used, and dispose of any waste materials properly.
By following these precautions, you can help ensure a safe and successful experiment while minimizing the risk of accidents or injury.
Lab Equipment Required for Flame Tests
In order to conduct heat-based chemical analysis effectively, specific tools and equipment are required. These materials ensure that the process is safe, accurate, and efficient. Proper equipment not only aids in the successful completion of the experiment but also helps maintain safety standards by controlling variables like temperature and exposure to chemicals.
Essential Equipment
The following list includes the core tools needed for this procedure. These instruments allow for the proper handling of samples and the safe application of heat to induce the desired reactions.
| Equipment | Purpose |
|---|---|
| Bunsen Burner | Provides a controlled heat source for heating samples. |
| Heat-Resistant Gloves | Protects hands from burns when handling hot materials. |
| Safety Goggles | Protects eyes from potential splashes or exposure to harmful fumes. |
| Wire Loops | Used to hold and dip the sample into the flame for heating. |
| Chemical Reagents | Substances being heated to observe color emissions. |
| Clamp or Stand | Holds the Bunsen burner or other heat sources in place. |
| Beaker or Dish | Holds liquid or solid substances for testing before heating. |
Additional Tools and Safety Equipment
Besides the primary equipment listed above, there are other tools that can be used to enhance the experiment’s safety and accuracy. Items such as fire extinguishers, emergency eyewash stations, and fume hoods can help manage unforeseen situations and provide additional protection during the experiment.
Flame Test vs Other Identification Methods
When identifying elements or compounds, various methods can be employed, each offering its own strengths and limitations. The procedure that involves heating substances to observe characteristic color changes provides a rapid way to detect certain elements. However, there are other techniques that may offer more detailed or accurate results depending on the context of the analysis. Below is a comparison of this method with several common alternatives.
- Colorimetric Analysis: This method involves detecting changes in color when a chemical reacts with a specific substance. While the color change in heating methods is due to the emission of light by heated elements, colorimetric methods can sometimes offer more quantitative results, especially when precise concentrations need to be determined.
- Spectroscopic Techniques: Methods like atomic absorption spectroscopy (AAS) and emission spectroscopy analyze the wavelengths of light absorbed or emitted by atoms or ions. Unlike the heat-based method, which provides a visual indication, these techniques allow for highly accurate identification and can detect elements in extremely small amounts.
- Chromatography: Used to separate the components of complex mixtures, chromatography can help identify various substances in a compound. This method is particularly useful when multiple elements are present in a sample, whereas heat-based methods may struggle to distinguish between similar elements in such mixtures.
- Electrochemical Methods: Techniques like potentiometry or voltammetry measure the electrical properties of a substance in solution, providing information about its chemical composition. These methods can offer detailed insights, especially for ionic compounds, which may not be easily analyzed using heating methods.
Although the heating procedure is fast and visually intuitive, other identification techniques often provide more precision, allow for the analysis of more complex mixtures, and can detect trace elements that might go unnoticed through visual observation. The selection of method typically depends on the specific requirements of the experiment and the level of detail necessary for the analysis.
Factors Affecting Flame Test Accuracy
The accuracy of identifying elements through color reactions can be influenced by several factors, which must be considered to ensure reliable results. While this method offers a straightforward and visual means of identification, various external conditions can affect how well the outcomes reflect the true composition of the sample. Understanding these factors can help minimize errors and improve the precision of the analysis.
Sample Purity: The presence of impurities or mixed substances can interfere with the distinct color emission of the element being analyzed. When multiple compounds are present, their reactions might overlap, leading to misleading results. Ensuring a pure sample is critical for accurate identification.
Temperature Control: The temperature at which a substance is heated plays a significant role in determining the color produced. Too high or too low a temperature may result in altered or incomplete reactions, which can distort the observed color. Maintaining a consistent heat source is important to achieve reliable results.
Equipment Calibration: Instruments used in the analysis, such as the burner or heat source, need to be well-calibrated. If the flame is too intense or not uniform, the results may be inconsistent. Proper calibration of the equipment ensures that the heat applied to the sample is appropriate for the reaction.
Environmental Factors: Factors such as the surrounding lighting conditions and air currents can also affect the visibility of the color produced during the analysis. A brightly lit environment or airflow near the sample may distort or diminish the color intensity, leading to incorrect conclusions.
Elemental Concentration: The concentration of the element in the sample directly influences the intensity of the color produced. Low concentrations may result in weak or undetectable color emissions, which could make identification more difficult. Conversely, very high concentrations could lead to overly intense colors, making it harder to distinguish between elements.
By carefully controlling these variables and ensuring the proper setup, the accuracy of identifying elements based on their characteristic color emissions can be significantly improved. This makes it a more reliable and effective technique for elemental analysis when used under the right conditions.
Importance of Flame Test in Qualitative Analysis
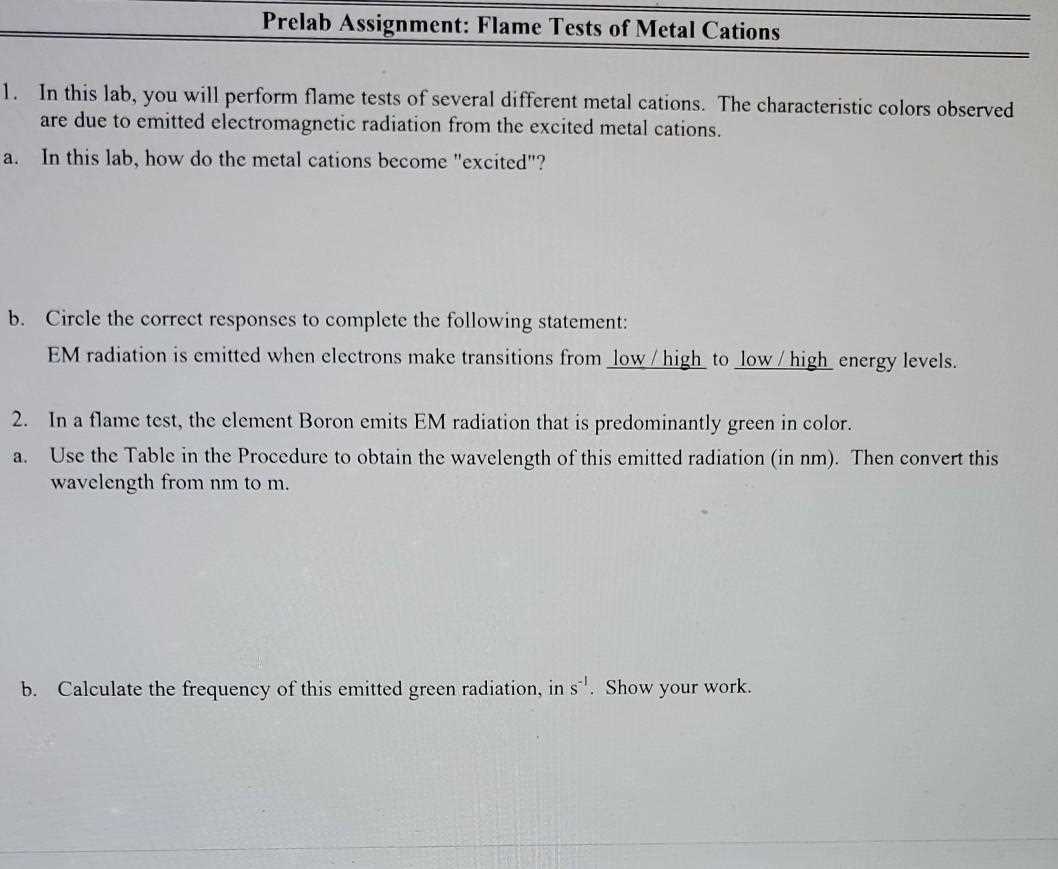
In qualitative analysis, identifying the composition of a substance is crucial for understanding its properties and behavior. One of the simplest and most effective methods for determining the presence of specific elements is through the observation of their characteristic color reactions when subjected to heat. This technique plays an important role in various scientific fields, offering a quick and reliable way to gain insights into the chemical makeup of a sample.
Rapid Identification: The ability to quickly identify elements based on their color emissions allows chemists to make immediate conclusions about the sample’s composition. This makes the method particularly useful in situations where fast analysis is needed, such as in educational settings or initial stages of research.
Complementary Tool: While the technique is not always precise enough for quantitative analysis, it serves as an excellent supplementary tool for other methods. When combined with more advanced techniques, this approach can help narrow down possibilities, guiding further investigation and confirming hypotheses.
Cost-Effective and Accessible: Unlike some complex analytical methods that require expensive equipment and highly trained specialists, this method is both affordable and easy to perform. It can be used in most laboratory environments without the need for specialized instruments, making it accessible to a wide range of professionals, from students to researchers.
Educational Value: For students and learners, this method provides an excellent introduction to the principles of qualitative analysis. It allows them to visualize the link between a substance’s composition and its physical properties, enhancing their understanding of chemistry and the scientific method.
Overall, while this approach may not replace more precise methods, it remains a vital part of qualitative analysis, providing valuable insights with minimal equipment and time investment. Its simplicity and effectiveness make it an indispensable tool for both educational and practical applications in chemistry.
Flame Test Experiment Procedure
When conducting an experiment to identify specific elements within a sample, the procedure involves several key steps to ensure accurate and reliable results. This process primarily relies on observing the color emitted by a substance when exposed to high heat, which reveals the presence of particular elements based on their unique emission spectra.
The following steps outline the typical procedure for performing the experiment:
- Prepare the Sample: Begin by preparing a small sample of the substance you wish to analyze. It can be in the form of a solid, liquid, or solution. If necessary, dissolve the sample in a suitable solvent.
- Clean the Equipment: Ensure that all equipment, including the wire loop, is clean to avoid contamination. Use a clean cotton swab and deionized water to wipe any residue from previous tests.
- Heat the Sample: Place a small portion of the sample on a clean metal wire loop and hold it in the flame of a Bunsen burner or other suitable heat source. The sample should be exposed to the flame long enough to produce a consistent color emission.
- Observe the Color: Observe the color of the flame as the sample is heated. The emitted color will correspond to the specific element present in the sample. Record the observed color and note any variations.
- Repeat the Process: For accuracy, perform the procedure several times using different samples or solutions to verify the consistency of the results.
- Document Results: After completing the experiment, document the colors observed for each sample. Compare the colors with known emission spectra for the identification of the elements present.
By following these steps, you can effectively perform the experiment to identify elements based on the colors they emit when subjected to high heat. This procedure, while simple, offers a reliable means of preliminary analysis in various laboratory settings.
Common Mistakes to Avoid in Flame Tests
During the process of identifying elements based on their emitted colors when exposed to heat, several common errors can lead to inaccurate or inconclusive results. Being aware of these mistakes can help improve the reliability and precision of the experiment. Avoiding these missteps is crucial for obtaining consistent and valid data, ensuring that the observed outcomes reflect the true nature of the sample being analyzed.
Below is a list of common mistakes to avoid when conducting such an experiment:
| Mistake | Impact on Results | How to Avoid |
|---|---|---|
| Using contaminated equipment | Residual chemicals can alter the color produced, leading to incorrect identification. | Always clean the equipment thoroughly between samples to avoid contamination. |
| Incorrect flame temperature | Too low a temperature may not excite the elements properly, and too high a temperature could cause interference from other substances. | Ensure that the flame temperature is adjusted to the optimal level for the sample being tested. |
| Insufficient sample quantity | Too little of the sample may not produce a visible color, making it difficult to identify the element. | Use an adequate amount of sample to ensure clear and observable color emission. |
| Not recording results properly | Failure to document colors or elements can lead to confusion and difficulty in analysis. | Take detailed notes on the color observed and the type of sample used for accurate comparison later. |
| Ignoring external light sources | Bright lights or distractions can obscure the flame’s color, making it hard to observe the true emission. | Conduct the experiment in a dimly lit room or block out external light sources. |
By avoiding these common mistakes, you can improve the accuracy of your analysis and ensure that the results are more reliable. Following the proper procedure and maintaining awareness of potential issues is key to successful experiments in this area.
Understanding the Role of Metal Ions
Metal ions play a pivotal role in many chemical reactions, particularly those that involve the interaction of substances with heat. When exposed to energy in the form of heat, metal ions absorb this energy, which excites their electrons. As the electrons return to their ground state, they release energy in the form of light, which is visible as different colors. This phenomenon forms the basis of identifying various elements based on their characteristic light emissions.
The Interaction of Metal Ions with Energy
When metal salts are heated, the metal ions undergo a process known as electronic excitation. This involves the movement of electrons to higher energy levels. As the electrons fall back to their lower energy states, they emit light. The wavelength (or color) of this emitted light is directly related to the energy difference between the excited state and the ground state of the electron. This means that each metal ion produces a unique color, which can be used as a fingerprint for that particular element.
Why Metal Ions Matter in Identification
Different metal ions emit light at specific wavelengths, which correspond to distinct colors. For example, lithium produces a red color, while copper may emit a greenish-blue hue. The precise color emitted depends on the particular element’s electron configuration and the energy levels available for excitation. By examining the color produced when these ions are exposed to heat, scientists can identify the presence of specific metals in a sample, making metal ions a crucial tool in qualitative analysis.
In summary, the unique light emitted by metal ions when heated makes them essential for element identification. Understanding their behavior allows chemists to interpret and identify substances with greater accuracy, which is fundamental to various scientific applications, from analytical chemistry to material science.