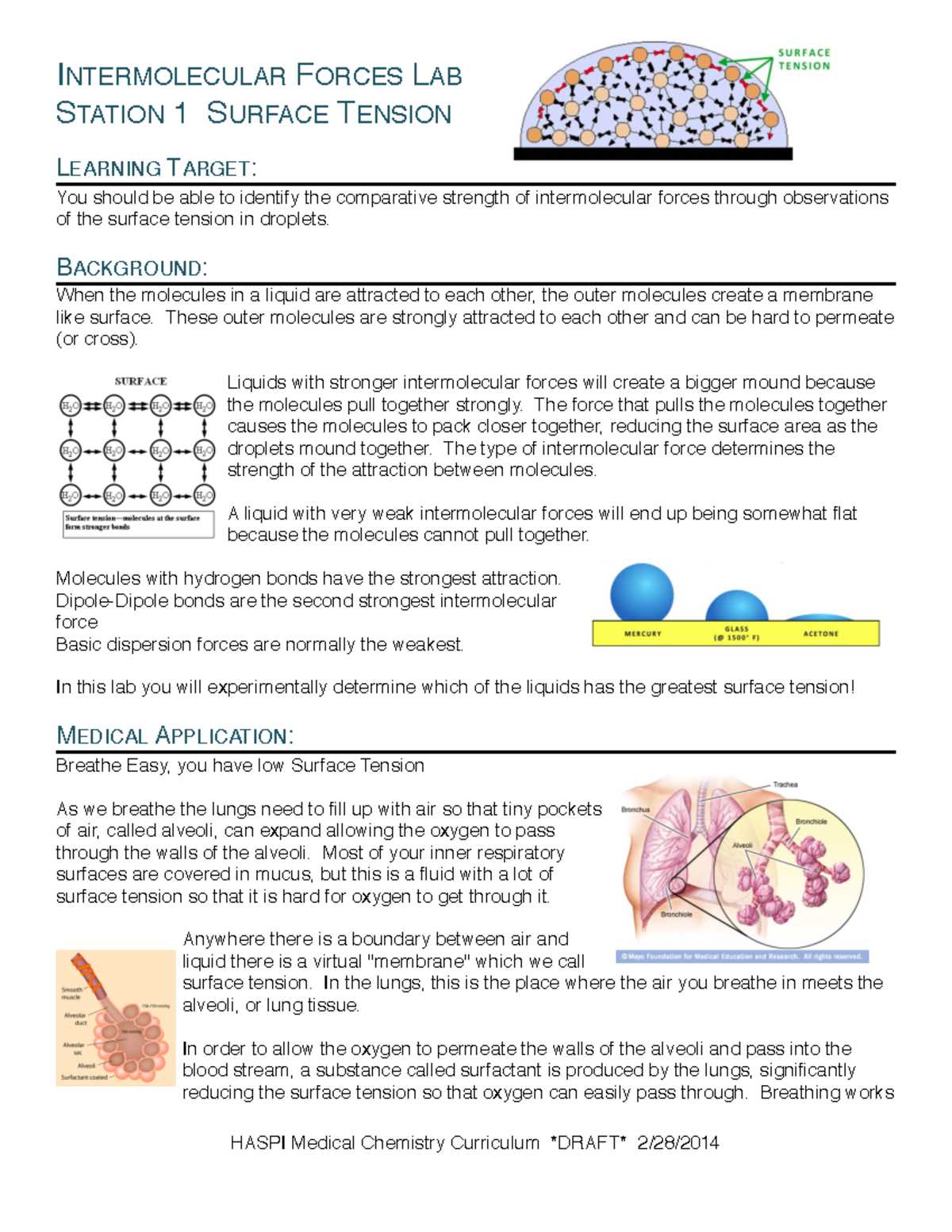
In scientific experiments, understanding the interactions between molecules is crucial for explaining various physical and chemical phenomena. These interactions are central to numerous processes, from the boiling and freezing points of substances to the properties of liquids and solids. By analyzing these relationships, we gain deeper insights into the behavior of matter under different conditions.
Various types of molecular attractions play a significant role in determining how substances react to changes in temperature, pressure, and other environmental factors. Each type of attraction affects the physical state of matter, influencing everything from viscosity to surface tension. These concepts form the foundation of many experimental setups aimed at exploring molecular behavior.
This section will explore key experiments that highlight the fundamental interactions between particles, offering a comprehensive analysis of the results. The aim is to clarify the underlying principles, ensuring a clearer understanding of the observed outcomes and their broader implications.
Lab 4 Molecular Interactions Insights
Understanding the behavior of substances at the molecular level is essential for grasping the underlying principles of various chemical reactions. In this section, we explore the relationships between particles, focusing on how different types of attractions influence the physical properties of materials. The results from the experiment help clarify how these interactions impact the state and behavior of matter.
The experiment examines several types of molecular attractions, comparing their strengths and effects on different substances. By analyzing the experimental outcomes, we can better understand which interactions dominate in various scenarios and how they contribute to observable changes in properties such as boiling points, viscosity, and solubility.
Through a detailed breakdown of the findings, this section aims to provide clarity on the various molecular behaviors observed during the experiment. The insights gathered will offer a deeper understanding of how molecules interact with each other and the practical implications of these interactions in everyday life.
Overview of Molecular Interactions
At the core of many chemical and physical processes, the interactions between molecules determine how substances behave under various conditions. These attractions are responsible for a wide range of phenomena, from the boiling and freezing points of liquids to the structure of solids. Understanding these relationships provides insight into the fundamental properties of matter.
There are different types of molecular attractions, each with unique characteristics and strengths. These forces govern how molecules interact with each other, influencing properties such as solubility, surface tension, and elasticity. By studying these interactions, we gain a better understanding of how materials respond to changes in temperature, pressure, and other environmental factors.
This section aims to provide a clear overview of the various types of molecular interactions and their effects on the behavior of substances. By examining the principles behind these attractions, we can better understand the underlying mechanisms that drive many natural and industrial processes.
Key Concepts in Molecular Interactions
Understanding the principles behind how molecules attract or repel each other is crucial for explaining the behavior of matter in different states. These concepts form the foundation for many chemical and physical processes, from the formation of liquids and solids to the behavior of gases. Below are the key ideas that guide our understanding of molecular interactions.
- Polarity: Molecules can be polar or non-polar, depending on how their atoms are arranged and the distribution of charge. This property significantly influences how they interact with other molecules.
- Electronegativity: The tendency of atoms to attract electrons in a bond. This property plays a crucial role in determining the strength of attractions between molecules.
- Dipole Moments: The presence of partial positive and negative charges within a molecule, influencing how molecules with opposite charges interact.
- Bonding Types: Different types of bonds, such as covalent and ionic, affect how molecules interact with each other. Understanding the differences helps explain various material properties.
By examining these concepts, we can predict how molecules will behave under various conditions. These fundamental ideas are essential for understanding everything from the behavior of water to the formation of complex biological structures.
Types of Molecular Interactions Explained
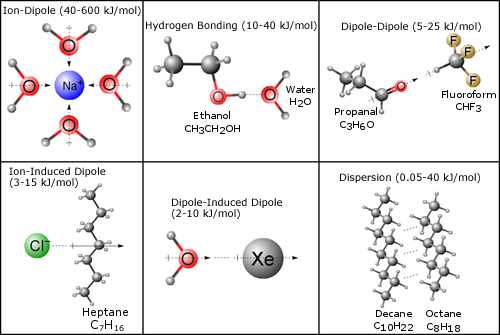
The interactions between molecules vary in strength and nature, influencing the properties of substances in fundamental ways. These interactions can be classified into several types, each with unique characteristics that determine how molecules behave under different conditions. Below are the main categories of molecular interactions.
- Hydrogen Bonding: A strong attraction that occurs between molecules where hydrogen is bonded to a highly electronegative atom, such as oxygen or nitrogen. This interaction is particularly important in water and biological systems.
- Dipole-Dipole Interactions: These interactions occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another. This type of attraction is common in substances like hydrochloric acid.
- London Dispersion Forces: The weakest form of attraction, arising from temporary fluctuations in electron density that induce momentary dipoles. While weak, these interactions are present in all molecules, especially in noble gases and non-polar molecules.
- Ion-Dipole Interactions: These occur when ions are attracted to the partial charges on polar molecules. This is particularly important in the dissolution of salts in water.
Each of these interactions plays a crucial role in determining how substances behave, from the structure of liquids to the formation of crystals and complex molecules. By understanding the various types of molecular interactions, we gain a better insight into the nature of matter itself.
Understanding Hydrogen Bonding in Lab 4

Hydrogen bonding is one of the most significant types of molecular interactions observed during experiments. It occurs when hydrogen atoms, bonded to electronegative elements like oxygen or nitrogen, form attractions with lone pairs of electrons on other electronegative atoms. This type of bonding plays a crucial role in determining the physical properties of many substances, particularly water, and is central to understanding the behavior of certain compounds in this experiment.
In the context of the experiment, hydrogen bonding influences various properties such as boiling points, solubility, and viscosity. The strength of these bonds affects how molecules arrange themselves and interact with one another, leading to observable results in different materials under study. Below is a table summarizing the key aspects of hydrogen bonding in the experiment:
| Property | Observation |
|---|---|
| Boiling Point | Substances with stronger hydrogen bonds tend to have higher boiling points due to the energy required to break the bonds. |
| Solubility | Compounds that can form hydrogen bonds with water are more likely to dissolve in it. |
| Viscosity | Stronger hydrogen bonding often results in higher viscosity, as molecules resist movement relative to one another. |
By analyzing the results of hydrogen bonding interactions, we can predict and explain the behavior of different substances in terms of their physical properties. This understanding is essential for interpreting the outcomes of the experiment and for applying these principles to real-world scenarios.
Dipole-Dipole Interactions and Examples
Dipole-dipole interactions occur between molecules that have permanent dipoles, meaning they possess regions of partial positive and partial negative charges due to differences in electronegativity between atoms. These attractions arise when the positive end of one molecule interacts with the negative end of another, and they play a significant role in shaping the physical properties of substances. Molecules that exhibit dipole-dipole interactions tend to have higher boiling points and increased solubility in polar solvents.
Characteristics of Dipole-Dipole Interactions

- Polar Molecules: Molecules with a permanent dipole moment have uneven charge distribution, leading to attractive interactions between opposite charges of adjacent molecules.
- Strength of Attraction: The strength of dipole-dipole interactions depends on the polarity of the molecules and the distance between them.
- Impact on Physical Properties: These interactions influence properties such as boiling points, melting points, and solubility in polar solvents.
Examples of Dipole-Dipole Interactions
- Hydrogen Chloride (HCl): The polar nature of HCl leads to strong dipole-dipole interactions, influencing its properties such as solubility in water and its relatively high boiling point for a small molecule.
- Acetone (CH3COCH3): The polarity of the carbonyl group in acetone leads to dipole-dipole attractions, contributing to its ability to dissolve in water and its moderate boiling point.
- Ammonia (NH3): Ammonia molecules interact through dipole-dipole forces, along with hydrogen bonding, which accounts for its relatively high boiling point and its behavior in aqueous solutions.
Understanding the role of dipole-dipole interactions helps explain the behavior of many common substances, providing insights into why certain compounds exhibit specific characteristics under various conditions. These interactions are fundamental to understanding how polar molecules interact with each other and with their environment.
London Dispersion Forces and Their Role
London dispersion interactions are a type of attraction that occurs due to temporary fluctuations in the electron distribution of molecules, creating momentary dipoles. Although these forces are relatively weak compared to other molecular attractions, they are present in all molecules, whether polar or nonpolar. These transient interactions play a significant role in the behavior of substances, particularly in gases and nonpolar compounds.
Despite their weakness, London dispersion forces are essential in explaining the properties of noble gases, nonpolar molecules, and substances with large atoms or molecules. As the size of the molecule increases, so does the strength of these interactions, influencing properties such as boiling and melting points. These interactions are especially noticeable in large, nonpolar molecules like iodine or in gases like helium.
The role of London dispersion forces extends to a variety of materials and systems. In nonpolar substances, where no permanent dipoles exist, these temporary interactions are the primary means of attraction between molecules. They also contribute to the ability of certain substances to condense from gases to liquids under the right conditions, even in the absence of stronger attractions like hydrogen bonds or dipole interactions.
Identifying the Strongest Forces in Lab 4
In any experiment involving molecular interactions, determining which types of attractions are the strongest can help explain the observed physical properties of substances. The strength of these interactions directly affects characteristics like boiling and melting points, solubility, and volatility. In this section, we will identify and compare the strongest interactions based on the compounds tested.
Comparison of Molecular Interactions
The strength of molecular attractions varies depending on the type of bonding involved. Here is an overview of how different types of interactions rank in terms of their strength:
- Hydrogen Bonding: Generally the strongest of the molecular attractions, hydrogen bonds form between molecules where hydrogen is directly bonded to highly electronegative atoms like oxygen or nitrogen.
- Dipole-Dipole Interactions: Polar molecules experience moderate attractions between the positive and negative ends of adjacent molecules. These forces are typically weaker than hydrogen bonds but still significant in affecting a substance’s properties.
- London Dispersion Forces: These are the weakest interactions, arising from temporary shifts in electron density. While individually weak, they are still present in all molecules and can become significant in larger molecules with more electrons.
Examples of Strong Interactions in the Experiment
In the compounds tested during the experiment, hydrogen bonding was found to be the dominant force in those that exhibited higher boiling points and stronger solubility in polar solvents. On the other hand, substances with predominantly London dispersion forces, such as nonpolar gases, displayed lower boiling points and less solubility in water.
Understanding which interactions are the most prominent allows for more accurate predictions of a substance’s behavior, and is crucial for interpreting the results of molecular experiments.
Methodology for Testing Molecular Interactions
The process of evaluating molecular attractions involves a series of well-defined steps designed to isolate and measure the influence of different types of molecular interactions on the properties of substances. By analyzing how molecules interact under various conditions, we can determine the strength and impact of these attractions on physical characteristics such as boiling points, solubility, and viscosity. This methodology provides valuable insights into the behavior of different compounds and the role that molecular interactions play in their properties.
In testing molecular interactions, the key lies in observing how molecules behave when exposed to different environmental factors such as temperature, pressure, or solvent type. These factors can reveal the strength of the attractions between molecules, allowing for an assessment of their relative significance. Below is a brief overview of the general steps involved in testing molecular interactions:
- Selection of Samples: Choose a range of substances that are likely to display different types of molecular interactions, such as polar, nonpolar, or hydrogen-bonding molecules.
- Measurement of Physical Properties: Conduct tests to measure boiling points, melting points, and solubility in various solvents. These properties can provide indirect evidence of the type and strength of molecular attractions present in the substances.
- Observations under Different Conditions: Examine how substances behave when subjected to changes in temperature or solvent conditions. The way substances react under these changes can help identify the dominant molecular interactions.
- Comparison of Results: Analyze the data to identify patterns or correlations between molecular structure and the observed properties. This comparison can help confirm which types of molecular interactions are most significant.
By carefully following these steps, it is possible to build a comprehensive understanding of the molecular interactions that govern the behavior of substances, providing insights that can be applied to real-world scenarios and further experimental investigations.
Results and Key Findings
The results obtained from the tests provided valuable insights into the nature of molecular interactions and their influence on the physical properties of different substances. By comparing various samples, we were able to determine which interactions were dominant in each compound and how these attractions affected key characteristics such as boiling points, solubility, and viscosity. This section outlines the main findings and observations drawn from the experimental data.
Observations on Physical Properties
The substances tested displayed a clear correlation between their molecular structure and the observed physical properties. For instance, compounds with stronger attractions, such as hydrogen bonds, exhibited significantly higher boiling points compared to those where only weak London dispersion interactions were present. In contrast, nonpolar molecules, which mainly rely on dispersion forces, had lower boiling points and were less soluble in polar solvents.
Analysis of Molecular Behavior
- Strong Attractions: Molecules with hydrogen bonding capabilities, such as alcohols and water, demonstrated stronger intermolecular interactions, leading to higher boiling points and greater solubility in water.
- Moderate Attractions: Polar molecules, such as acetone, exhibited moderate dipole-dipole interactions, resulting in moderate boiling points and higher solubility in polar solvents.
- Weak Attractions: Nonpolar compounds, like iodine or oxygen, showed weaker interactions, leading to lower boiling points and reduced solubility in polar solvents.
These findings confirm that the strength and nature of molecular interactions play a crucial role in determining the properties of substances. The results not only deepen our understanding of molecular behavior but also highlight the importance of molecular structure in predicting the physical characteristics of different compounds.
Analyzing the Impact of Temperature
Temperature plays a crucial role in determining the strength of molecular interactions and how substances behave under various conditions. As temperature increases, the kinetic energy of molecules also rises, which can affect how tightly or loosely molecules are held together. This section explores the effect of temperature on the physical properties of substances and how it influences molecular behavior, specifically focusing on changes in boiling points, solubility, and molecular motion.
In general, as temperature rises, the increased energy tends to weaken attractive forces between molecules, making it easier for molecules to move apart from each other. This leads to physical changes such as melting, evaporation, or increased solubility in some cases. Below is a summary of how temperature variations affected the properties of different substances during the experiments:
| Substance | Boiling Point (°C) | Effect of Increased Temperature |
|---|---|---|
| Water | 100°C | Boiling point remained stable but vaporized rapidly at higher temperatures due to stronger hydrogen bonds. |
| Acetone | 56°C | Boiling point lowered slightly with increasing temperature, showing weaker dipole interactions. |
| Iodine | 184°C | Evaporated faster with rising temperature due to weak London dispersion forces. |
The table above demonstrates how different compounds react to increased temperature. As expected, substances with stronger molecular interactions, such as water, showed little change in boiling point but exhibited faster vaporization, while compounds with weaker attractions like iodine and acetone experienced more noticeable changes in physical state and boiling point. Understanding the impact of temperature is vital for predicting how materials will behave in real-world applications, such as in the chemical industry or material science.
Effect of Molecular Size on Forces
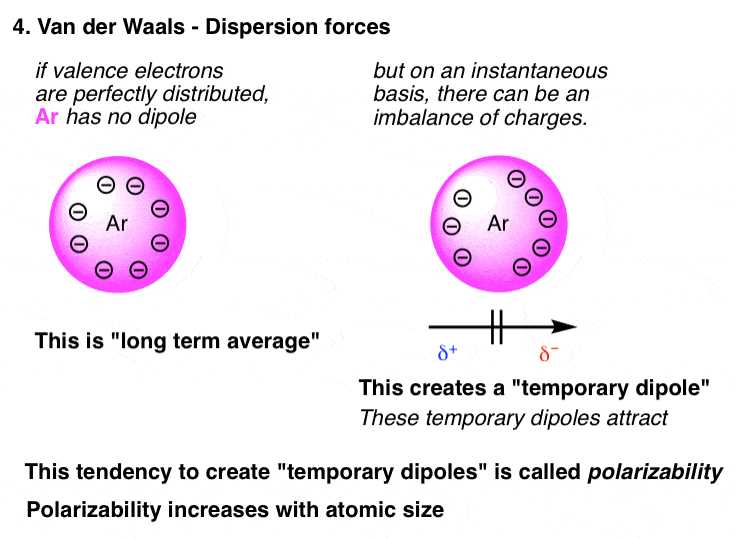
The size of a molecule significantly impacts the nature and strength of the interactions between its molecules. Larger molecules, with more atoms and electrons, tend to exhibit stronger interactions compared to smaller molecules. This is due to the increased surface area and greater number of electrons available for creating temporary dipoles or other attractions. In this section, we examine how the size of molecules influences the strength of the attractive interactions and how these interactions affect the physical properties of substances.
Larger Molecules and Stronger Interactions
As molecules increase in size, their ability to form temporary dipoles also grows. These temporary shifts in electron density lead to stronger dispersion interactions, which are more significant in larger molecules. For example, long-chain hydrocarbons have stronger attractions than smaller molecules because of the increased number of electrons, leading to higher boiling points and increased viscosity. The enhanced interaction is not only due to the size but also because larger molecules are less mobile, allowing these attractions to be more prominent.
Smaller Molecules and Weaker Interactions
On the other hand, smaller molecules have fewer electrons and a smaller surface area for interactions. As a result, their attractive forces are weaker, and they typically exhibit lower boiling points and higher volatility. For instance, smaller gases like hydrogen or nitrogen have weak dispersion interactions and do not require as much energy to break apart compared to larger molecules, leading to faster evaporation and lower melting points.
The relationship between molecular size and the strength of attractions is critical in determining how substances behave in different environments. Understanding these effects helps in predicting the physical behavior of materials under varying conditions, such as temperature and pressure, which is important in fields ranging from chemistry to material science.
Common Mistakes in Intermolecular Force Analysis
When analyzing molecular interactions, there are several common errors that can lead to inaccurate conclusions. These mistakes often arise from misunderstandings of the underlying principles or overlooking key factors that affect molecular behavior. In this section, we will explore some of the most frequent errors made during such analyses and discuss how to avoid them to ensure more accurate results.
Overlooking Molecular Geometry
One common mistake is failing to consider the geometry of molecules when analyzing their interactions. The shape and arrangement of atoms within a molecule can significantly influence the strength and type of attraction that occurs between molecules. For example, molecules with a more symmetrical shape may exhibit weaker dipole-dipole interactions, while asymmetrical molecules may lead to stronger interactions due to greater electron distribution imbalance.
Ignoring Temperature Effects
Another error is neglecting the influence of temperature on molecular interactions. Temperature affects the kinetic energy of molecules, which in turn can alter the strength of their interactions. For example, higher temperatures generally weaken attractions between molecules, leading to changes in phase transitions, such as melting or boiling points. Ignoring this factor can result in misinterpreting the strength or behavior of molecular interactions.
Misinterpreting the Role of Size

Size is a critical factor that can determine the strength of molecular interactions, but it is often misunderstood. Larger molecules typically have more electrons, leading to stronger dispersion interactions. However, some may overlook this effect and fail to recognize that larger molecules with greater surface area experience stronger attractions than smaller molecules with fewer electrons.
Incorrectly Applying Principles to All Molecules
Not all molecules behave in the same way, and applying general principles without accounting for the specific characteristics of each molecule is another common mistake. For instance, hydrogen bonding is not present in all polar molecules, and dispersion forces are particularly prominent in non-polar molecules. Applying the same rules across the board can lead to inaccurate conclusions.
| Mistake | Consequence | Solution |
|---|---|---|
| Ignoring molecular geometry | Leads to incorrect conclusions about interaction strength | Consider the shape and symmetry of molecules |
| Neglecting temperature effects | Misinterprets phase changes and behavior | Account for temperature in analysis |
| Misunderstanding molecular size impact | Underestimates the strength of interactions | Factor in molecular size when assessing attractions |
| Incorrect generalization of principles | Inaccurate analysis of different molecules | Apply specific principles to individual molecules |
By being mindful of these common mistakes, researchers can better analyze molecular interactions and improve their understanding of how molecules behave under different conditions. A more nuanced approach that takes into account the geometry, temperature, size, and specific characteristics of molecules can help ensure a more accurate and reliable analysis.
How to Interpret Experimental Data
When conducting experiments involving molecular interactions, accurately interpreting the collected data is essential to understanding the results and drawing meaningful conclusions. Proper data analysis involves not only looking at raw values but also considering the broader context, experimental design, and potential sources of error. In this section, we will outline key approaches and considerations for interpreting experimental results effectively.
Identifying Patterns in the Data
The first step in interpreting experimental data is to identify any observable trends or patterns. For example, you might observe a correlation between temperature and the strength of molecular interactions or changes in molecular structure and phase behavior. It is crucial to look for consistency in the data, as this can indicate a strong relationship or causal link between variables. Graphs and charts can be useful tools in visualizing these patterns and identifying outliers or irregularities.
Accounting for Experimental Variables
In any experimental setup, numerous variables can affect the outcome. Temperature, pressure, concentration, and molecular size are just a few factors that may influence the results. It is important to assess how these variables were controlled or measured during the experiment. For instance, if temperature was not properly maintained throughout the test, it could distort the data and lead to incorrect conclusions. Carefully reviewing the experimental methodology can help ensure that all variables were accounted for and that the results are valid.
In addition to external factors, consider the possibility of measurement errors or instrument limitations. Variations in equipment calibration or sample preparation can introduce discrepancies. Identifying and addressing these potential issues can help refine your interpretation and improve the accuracy of your conclusions.
Making Inferences Based on Results
Once the patterns and variables have been carefully analyzed, it’s time to make inferences based on the data. For example, if increasing temperature consistently weakens molecular attractions, you may infer that the strength of these interactions is temperature-dependent. However, it is essential to ensure that these inferences are supported by the data and that any claims are backed by sufficient evidence. Avoid jumping to conclusions without thorough analysis and always be open to alternative explanations.
Additionally, interpreting experimental data often requires comparing the results with theoretical models or previous studies. This comparison can help verify the validity of your findings and provide a deeper understanding of the underlying principles.
In summary, interpreting experimental data is an iterative process that involves recognizing patterns, controlling for variables, addressing potential errors, and making supported inferences. By following a structured approach, researchers can derive meaningful insights from their experiments and contribute to the advancement of knowledge in the field.
Applications of Intermolecular Forces
The interactions between molecules play a crucial role in a variety of scientific and industrial processes. These molecular attractions are fundamental to understanding the behavior of matter in different states and are integral to numerous applications in fields such as chemistry, biology, and materials science. In this section, we will explore how these molecular interactions impact everyday technologies and natural phenomena.
Influence on Physical Properties

Molecular attractions significantly affect the physical properties of substances, such as boiling and melting points, viscosity, and solubility. These properties are essential in numerous applications, from pharmaceuticals to food science. Understanding how molecules interact allows scientists to manipulate these properties to create new materials or improve existing ones. Examples include:
- Boiling and Melting Points: The strength of molecular interactions determines how much energy is required to change a substance’s state, which is critical in processes like distillation or crystallization.
- Solubility: The solubility of substances in solvents depends on the compatibility of their molecular interactions, influencing everything from drug formulation to the production of cleaning agents.
- Viscosity: The resistance to flow in liquids is influenced by how molecules interact with each other, important in industries such as lubricants, paints, and food production.
Applications in Medicine and Biotechnology
In the medical and biotechnological fields, understanding how molecules interact is essential for designing effective drugs, vaccines, and diagnostic tools. For example, drug molecules must bind to specific receptors in the body, a process governed by molecular interactions. Additionally, the creation of biomaterials for implants or prosthetics often relies on manipulating molecular forces to ensure compatibility and durability.
- Drug Delivery: Nanoparticles and liposomes are engineered to transport drugs to specific sites in the body by exploiting molecular interactions, enhancing therapeutic effectiveness.
- Vaccine Development: Vaccine formulations rely on molecular interactions to stabilize active ingredients and improve immune responses.
- Biomaterials: The design of biocompatible materials for medical implants depends on understanding how molecular forces affect interactions with biological tissues.
Role in Environmental and Energy Solutions

Molecular interactions also play a role in solving environmental challenges. For example, the development of materials for carbon capture and storage (CCS) relies on understanding how different molecules interact with CO2. Similarly, energy-efficient materials, such as those used in solar panels or batteries, often derive their efficiency from carefully engineered molecular attractions.
- Carbon Capture: Materials designed to capture CO2 from the atmosphere or industrial processes rely on specific molecular forces to selectively bind with carbon dioxide.
- Energy Storage: The efficiency of batteries and capacitors depends on molecular interactions within the materials used for energy storage and transfer.
In conclusion, molecular attractions are integral to a wide range of applications that affect our daily lives and the advancement of various industries. By studying and harnessing these interactions, scientists and engineers can continue to innovate in fields ranging from medicine to environmental protection.
Practical Tips for Lab 4 Success
Successfully completing an experiment requires not only understanding the theoretical concepts but also being well-prepared and methodical in your approach. By following a few key strategies, you can ensure accurate results and a smooth experience during your hands-on work. This section provides essential tips to maximize efficiency and reliability in your experimental process.
1. Prepare Thoroughly
Before beginning, make sure you have a clear understanding of the objectives and the steps involved. Review all relevant background information, including the concepts you will be testing and the techniques you’ll be using. Preparation is key to avoiding mistakes during the procedure and helps to troubleshoot issues if they arise.
- Review the theoretical principles to grasp how the experiment fits within the broader scientific context.
- Ensure that you understand the proper usage of equipment and materials.
- Make a checklist to confirm that all necessary items are available before starting.
2. Follow Protocols Carefully
Precision in following instructions is essential to obtaining valid results. Deviating from the provided procedures, even slightly, can lead to inaccurate or unreliable data. Stick closely to the steps outlined, and don’t hesitate to ask for clarification if anything is unclear.
- Read through the experimental steps multiple times before beginning.
- Use the appropriate quantities of chemicals and tools as instructed.
- Take note of any specific conditions (e.g., temperature or time) mentioned in the instructions.
3. Document Everything
Accurate record-keeping is crucial for both analyzing results and troubleshooting potential problems. Keep detailed notes of observations, measurements, and any deviations from the expected procedure. This documentation will be invaluable when reviewing your findings and discussing any issues during analysis.
- Use a dedicated notebook or digital format to document every step and result.
- Record both expected and unexpected observations during the experiment.
- Note any adjustments made to the procedure and their impact on results.
4. Practice Safety and Organization
Always prioritize safety by following proper lab protocols. Ensure that you have access to safety equipment such as gloves, goggles, and lab coats. Keeping your workspace organized will not only help prevent accidents but also save time during the experiment.
- Wear the appropriate protective gear at all times.
- Keep work surfaces clean and free from unnecessary clutter.
- Know the location of emergency exits, eyewash stations, and first aid kits.
5. Analyze Data Carefully

Once the experiment is complete, carefully analyze the results. Look for patterns, outliers, or inconsistencies that might suggest an error or unexpected outcome. Double-check calculations and ensure that your data aligns with theoretical expectations. Use critical thinking to interpret your findings accurately.
- Compare your results with theoretical predictions or expected outcomes.
- Consider all possible sources of error, and assess their impact on your results.
- Present your data clearly using tables or graphs to make it easier to analyze.
By following these practical tips, you’ll be well-equipped to complete your experiment successfully and gain valuable insights from the process. A thoughtful and organized approach is essential for achieving reliable results and improving your scientific understanding.
Further Reading on Molecular Interactions
Understanding the behavior of molecules and the interactions between them is a crucial aspect of various scientific disciplines, including chemistry, biology, and material science. If you’re interested in delving deeper into this area, several resources can help you gain a more comprehensive understanding of the underlying principles. This section highlights key readings and references to explore for those looking to expand their knowledge of molecular behavior and its practical applications.
1. Fundamental Texts in Molecular Chemistry
For a foundational understanding of how molecules interact, several textbooks provide in-depth explanations of the forces at play. These texts cover both theoretical frameworks and experimental observations, helping readers grasp the core concepts behind molecular interactions.
- Principles of Chemistry by Peter Atkins and Loretta Jones – A comprehensive introduction to chemical principles, including molecular structure and bonding.
- Physical Chemistry by Peter Atkins and Julio de Paula – This book offers an advanced treatment of thermodynamics, quantum mechanics, and the molecular interactions that influence chemical behavior.
- Introduction to Molecular Chemistry by K. M. D. J. Howard – A more approachable text that breaks down key concepts for beginners and intermediate learners.
2. Research Articles and Journals

For those looking to explore the latest developments and detailed studies on molecular interactions, scientific journals are an excellent resource. These papers often focus on the cutting-edge research and experimental techniques used to study molecular behavior.
- Journal of Chemical Physics – A leading journal that publishes original research on the physical properties of molecules and their interactions.
- Nature Chemistry – This journal covers a broad spectrum of topics, including molecular dynamics and the role of molecular interactions in chemical processes.
- Annual Review of Physical Chemistry – A comprehensive review of advancements in the field of physical chemistry, with sections on molecular theory and the interaction of molecules in various environments.
By engaging with these readings, you’ll deepen your understanding of how molecules interact in different contexts and gain insight into the methods used to study these interactions. Whether you’re a student, researcher, or enthusiast, the resources listed here will provide you with the tools needed to explore this fascinating field further.