As you approach the end of your AP course, it’s crucial to refine your knowledge and sharpen your skills. This section will guide you through key strategies to succeed in the upcoming evaluation, ensuring you’re fully prepared to tackle every aspect with confidence.
From mastering core concepts to practicing problem-solving techniques, a structured approach to your studies will help you focus on the most important areas. With the right resources and strategies, you can effectively reinforce your understanding and optimize your performance.
Make use of study materials, practice tests, and review sessions to consolidate what you’ve learned. Stay organized, stay motivated, and focus on building both your content knowledge and test-taking skills to excel.
AP Chemistry Final Exam Review Plan

To achieve success in the upcoming assessment, it’s essential to have a well-organized study strategy. A structured approach allows you to target critical topics, refine your skills, and strengthen areas where you may need additional practice. This plan will help you prioritize your preparation and ensure you’re ready to face any challenge that comes your way.
Start by identifying the most important concepts from each unit. Focus on areas with the highest weight and those that have historically been more complex. Establishing clear goals for each study session will help you stay focused and track your progress.
Incorporate various study techniques, such as active recall, spaced repetition, and problem-solving practice. Mix in different resources, such as textbooks, online guides, and past questions, to ensure well-rounded preparation. Consistency and dedication are key, so stick to your schedule and gradually increase the intensity of your practice as the test approaches.
Understand the Exam Structure

To perform well in the upcoming test, it’s essential to have a clear understanding of its format. Knowing the structure of the assessment will help you allocate your time effectively and focus on the most relevant sections. Familiarity with how the questions are organized allows you to approach each part with a strategic mindset.
The test typically includes multiple-choice questions and free-response items. Each section tests different skills, from factual recall to complex problem-solving. The multiple-choice part often covers a broad range of topics, while the free-response section focuses more on detailed explanations and calculations.
It’s important to understand the weighting of each section, as this will guide how much time to dedicate to each. Knowing the time limits for each part will help you pace yourself and avoid spending too long on any single question. By practicing with sample papers, you can become more comfortable with the format and improve your test-taking efficiency.
Key Topics to Focus On
To maximize your chances of success, it’s important to concentrate on the most crucial subjects. Identifying the key areas that are frequently tested will allow you to streamline your study efforts and ensure you are well-prepared for all types of questions. Focusing on these topics will give you a strong foundation to build on.
Core Concepts and Principles
Pay particular attention to the fundamental concepts, such as atomic structure, bonding, and the periodic table. These are foundational ideas that support many other sections. A deep understanding of these principles will help you answer a wide range of questions accurately and efficiently. Practice applying these concepts to real-world scenarios to strengthen your grasp.
Problem-Solving and Calculations
Another essential area is mastering the various types of calculations, including stoichiometry, thermodynamics, and equilibrium. These problems require not only memorization but also the ability to apply formulas and principles to different situations. Focus on practicing these types of questions to improve your speed and accuracy under test conditions.
Time Management During the Test
Effective time management is crucial for achieving a high score. Knowing how to allocate your time wisely during the assessment can make a significant difference in your performance. Proper planning helps ensure that you have enough time to answer all questions thoroughly without rushing through any sections.
Setting Time Limits for Each Section
Before starting, divide your available time based on the number of sections and their weight. For example, allocate more time to complex tasks, like problem-solving or free-response questions, and less to simpler multiple-choice items. By setting specific time limits for each part, you can avoid spending too much time on any one section and ensure you finish the test.
Stay Flexible and Adjust if Needed
Even with a plan, be prepared to adjust your timing as you progress. If you find yourself struggling with a question, don’t waste excessive time on it. Move on and come back to it later if possible. Prioritize answering questions you’re confident in first, and save challenging ones for the end when you have a clearer head. Staying flexible with your strategy can help you remain calm and focused throughout.
Mastering Organic Chemistry Concepts
To succeed in the section covering organic compounds, it’s essential to fully understand the underlying principles and mechanisms. This topic often requires you to apply both theoretical knowledge and practical problem-solving skills. By mastering key concepts and practicing various types of questions, you can improve both your speed and accuracy when tackling related problems.
Key Areas to Focus On
Focus on the following core concepts that are frequently tested:
- Functional Groups: Understanding the behavior and properties of different functional groups is critical.
- Reaction Mechanisms: Learn the steps and patterns behind common reactions, such as substitution and elimination.
- Isomerism: Study the different types of isomers and how they impact molecular behavior.
- Synthesis Pathways: Practice designing synthetic routes based on given reagents and conditions.
Approach to Problem Solving
When working on organic problems, break them down into manageable steps:
- Identify the type of reaction or concept being tested.
- Write out all known information and draw any structures if necessary.
- Apply the relevant mechanisms or rules to solve the problem.
- Double-check your answer for logical consistency and correctness.
By practicing regularly and mastering these areas, you’ll be well-prepared to tackle any question related to organic compounds with confidence.
Essential Equations for the Test

For success in the upcoming assessment, it’s crucial to be well-versed in the key formulas and equations that will be used throughout the test. Understanding how and when to apply these formulas can significantly impact your ability to solve problems quickly and accurately. Mastering these equations will give you the confidence to tackle a wide range of questions.
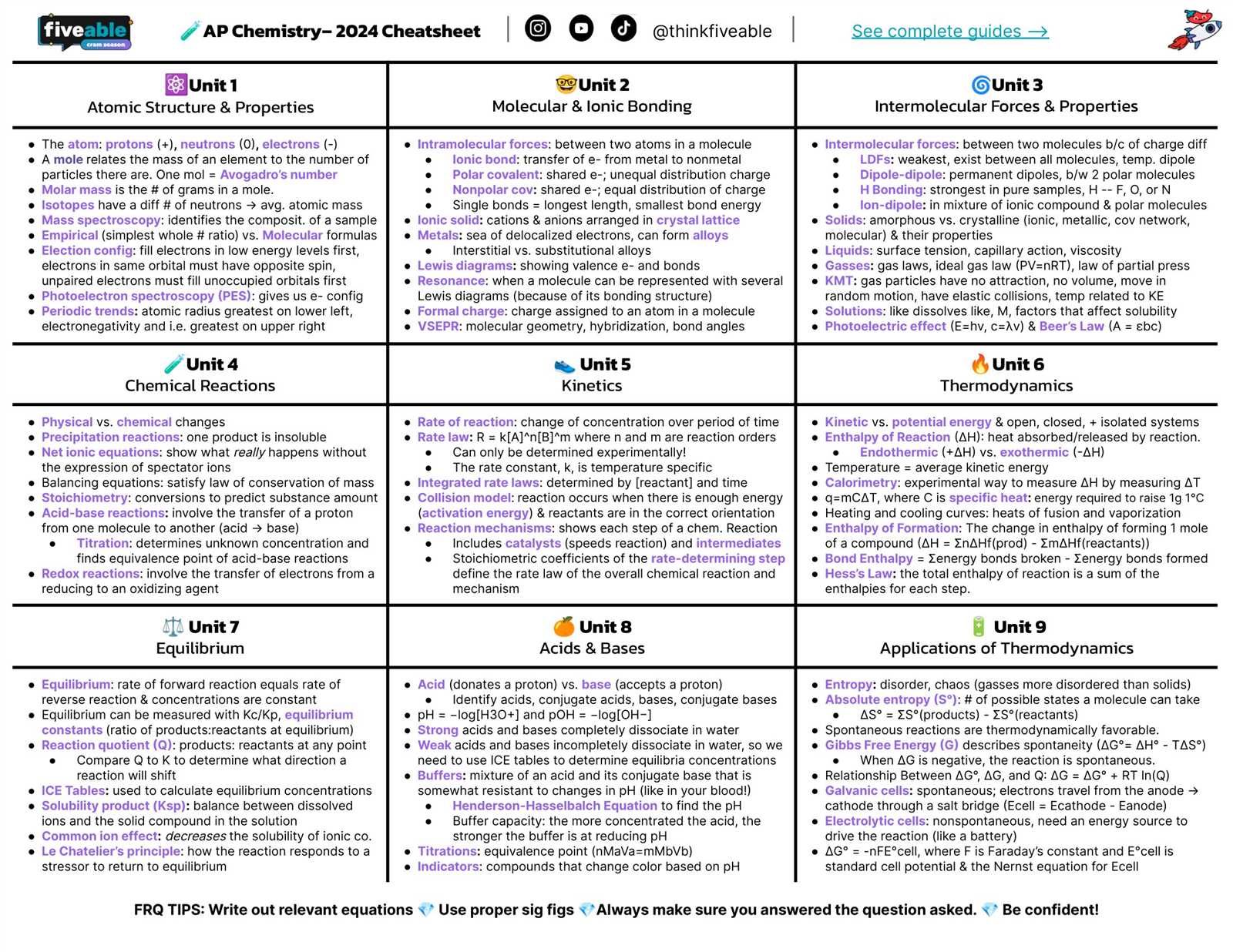
Core Formulas to Memorize

Focus on the following essential equations that you’ll likely encounter:
- Ideal Gas Law: PV = nRT – Understand how to use this equation for problems involving gases.
- Stoichiometry: moles = mass / molar mass – Useful for converting between mass, moles, and particles.
- Concentration: M1V1 = M2V2 – Essential for dilution calculations and concentration changes in solutions.
- Energy Change: q = mcΔT – Important for calculating heat changes in a substance during temperature changes.
Commonly Used Relationships
In addition to the core equations, there are several other relationships that you will frequently use during problem-solving:
- Reaction Rate: Rate = k[A]^n – Understand how the rate constant and concentration affect reaction speed.
- Equilibrium Constant: K = [products] / [reactants] – Essential for understanding chemical equilibrium and its shifts.
- Work and Energy: W = Fd – Fundamental in thermodynamics and energy transfer calculations.
Being comfortable with these equations will not only save you time during the test but also help you confidently approach complex problems. Practice applying them regularly to reinforce your understanding and ensure accuracy under timed conditions.
Practice with Past Exam Papers
One of the most effective ways to prepare for the upcoming test is to practice with previous assessment papers. Working through past questions helps you become familiar with the format, improves your problem-solving speed, and highlights areas where you may need more practice. It’s also a great way to gauge the types of questions that are most commonly asked.
By simulating real test conditions and practicing under timed constraints, you can reduce test anxiety and improve your time management skills. It’s also beneficial to review the solutions after completing each set to understand your mistakes and reinforce your learning.
Benefits of Using Past Papers
| Benefit | Explanation |
|---|---|
| Familiarity with Question Format | Helps you understand the structure of questions and common themes. |
| Improved Problem-Solving Skills | Enables you to practice applying concepts to a variety of scenarios. |
| Time Management | Allows you to practice pacing yourself and avoiding time-related stress. |
| Identifying Knowledge Gaps | Reveals areas that may need further study and attention. |
Incorporating past questions into your study routine will make you more confident and prepared for the upcoming assessment. Be sure to analyze your answers and take note of any recurring themes or question types to optimize your study plan.
Effective Use of Study Guides

Study guides are invaluable tools that can help streamline your revision process. By consolidating key information, they allow you to focus on the most important topics and improve your understanding of complex material. When used correctly, study guides can enhance retention and make your preparation more efficient and targeted.
Rather than passively reading through notes or textbooks, a study guide offers a structured approach to review. It helps break down material into manageable chunks, so you can focus on mastering individual concepts before moving on to more advanced topics. The key is to actively engage with the guide by taking notes, highlighting important points, and testing yourself regularly.
How to Maximize the Use of Study Guides
| Strategy | Benefit |
|---|---|
| Break It Down Into Sections | Divide the guide into smaller topics to ensure thorough understanding of each part. |
| Use Active Recall | Test yourself regularly on the material to improve memory retention and active learning. |
| Focus on Weak Areas | Identify and spend more time on sections where you feel least confident. |
| Review Regularly | Consistent review helps reinforce the material and prevents cramming. |
By following these strategies and consistently using study guides throughout your revision, you’ll be able to refine your understanding and approach to the upcoming assessment with confidence and clarity.
Tips for Multiple-Choice Questions
Multiple-choice questions are designed to test your understanding of a wide range of topics in a concise format. To perform well, it’s important to apply specific strategies that allow you to quickly analyze each question and eliminate incorrect answers. Mastering these techniques can significantly improve your accuracy and efficiency during the assessment.
One key strategy is to carefully read each question and all available options before making your selection. Often, multiple-choice questions are crafted to include subtle clues that can help you identify the correct answer. Additionally, if you’re unsure about a question, it’s beneficial to use the process of elimination to rule out obviously incorrect choices.
Strategies for Success
- Read the Question Thoroughly: Ensure you understand what is being asked before considering the answer choices.
- Eliminate Incorrect Options: Cross out answers that are clearly wrong to narrow down your choices.
- Watch for Keywords: Look for keywords or phrases that are commonly used in correct answers, such as “always,” “never,” or “best.”
- Don’t Rush: Take your time to think through each question, especially if it seems tricky. Moving too quickly can lead to mistakes.
By incorporating these strategies into your approach, you can tackle multiple-choice questions with greater confidence and improve your performance throughout the test.
Approaching Free Response Questions
Free response questions require you to demonstrate a deeper understanding of concepts and apply your knowledge to complex problems. Unlike multiple-choice questions, these require a more thorough and detailed answer, often with calculations, explanations, and logical reasoning. To perform well, it’s essential to approach these questions strategically and methodically.
When tackling free response questions, it’s important to stay organized. Begin by carefully reading the question to ensure you understand exactly what is being asked. Break the problem into smaller parts and address each one step by step. This will help you stay focused and ensure that you cover all necessary aspects of the question.
Effective Strategies for Free Response
- Read the Question Carefully: Ensure you understand the problem before starting to write. Look for specific instructions, units, or details that need to be included in your response.
- Plan Your Answer: Before writing, outline your approach. List the steps you’ll take to solve the problem, and make sure each part of your response addresses the key elements of the question.
- Show All Work: Always provide a clear explanation of your thought process. Even if the final answer is correct, showing your steps can earn partial credit in case of a mistake.
- Use Units and Significant Figures: Be meticulous about including correct units and applying proper significant figures in calculations. This attention to detail can make a difference in your score.
- Stay Organized: Write your answer neatly and in a logical order. This helps ensure clarity and makes it easier for the grader to follow your reasoning.
By following these strategies, you can approach free response questions with confidence and increase your chances of earning the maximum possible score. Practice regularly and refine your skills to make sure you are fully prepared to tackle these types of questions effectively.
Reviewing Chemical Reactions and Mechanisms
Understanding the various types of reactions and the mechanisms behind them is crucial for mastering complex topics. These processes govern how substances interact and transform, and recognizing the patterns can make solving related problems more intuitive. To effectively approach these topics, it’s important to understand both the overall reaction pathways and the intermediate steps that lead to product formation.
Mastering reaction mechanisms involves learning the sequence of steps through which reactants turn into products. These steps often include the breaking and forming of bonds, which can be influenced by factors like temperature, concentration, and catalysts. A clear grasp of these mechanisms will help you predict the behavior of molecules and better understand the driving forces behind reactions.
Types of Reactions to Focus On

- Combustion: Reactions where a substance reacts with oxygen, releasing energy in the form of light or heat.
- Synthesis: The combination of simpler substances to form a more complex product.
- Decomposition: Reactions where a compound breaks down into simpler substances.
- Single and Double Displacement: Reactions where ions are exchanged between reactants to form new products.
- Redox Reactions: Reactions involving the transfer of electrons between substances, affecting their oxidation states.
Key Factors Affecting Reaction Mechanisms
- Concentration: Higher concentrations can increase the rate of reaction by providing more reactant particles for collisions.
- Temperature: Increased temperature generally speeds up reactions by raising the energy of the molecules involved.
- Catalysts: Substances that speed up reactions without being consumed, lowering the activation energy required for the process.
- Solvent: The medium in which a reaction occurs, which can influence the rate and pathway of the reaction.
By reviewing these concepts and practicing problems related to different reaction types and mechanisms, you will build a strong foundation to approach more complex scenarios with confidence and accuracy.
Understanding Thermodynamics and Kinetics
To master the behavior of systems during transformations, it’s essential to understand both the energetic and the rate aspects of reactions. Thermodynamics focuses on the energy changes associated with chemical processes, determining whether a reaction can happen spontaneously. On the other hand, kinetics explores the speed of these transformations and the factors that influence how quickly reactions occur. Together, these areas provide a comprehensive picture of how and why reactions happen.
While thermodynamics can tell you whether a reaction is favorable, kinetics reveals the pathway it follows and the factors that control the rate at which it reaches completion. Understanding both concepts allows for a deeper understanding of how substances interact and change under various conditions.
Key Thermodynamic Concepts
- Enthalpy: The heat content of a system, which can be released or absorbed during a reaction.
- Entropy: A measure of disorder or randomness in a system, with reactions tending to move towards increased entropy.
- Gibbs Free Energy: A combination of enthalpy and entropy that predicts the spontaneity of a reaction. If the value is negative, the process is thermodynamically favorable.
Factors Affecting Reaction Kinetics
- Activation Energy: The energy required to start a reaction, which must be overcome for the reaction to proceed.
- Concentration: Higher concentrations of reactants generally lead to more collisions and faster reactions.
- Temperature: Increasing the temperature typically increases the reaction rate by providing molecules with more energy to overcome activation barriers.
- Catalysts: Substances that lower the activation energy, speeding up reactions without being consumed in the process.
By understanding the balance between thermodynamic favorability and kinetic barriers, you can better predict the behavior of systems and how they will evolve over time. Mastering these concepts will allow you to tackle complex problems with greater precision.
Common Mistakes to Avoid
During assessments and problem-solving sessions, it’s easy to fall into certain traps that can lead to errors. Recognizing these common pitfalls and learning how to avoid them is key to improving your performance. Many mistakes stem from overlooking details, misinterpreting questions, or failing to apply concepts correctly. By becoming aware of these common issues, you can sharpen your approach and increase your chances of success.
Below are some of the most frequent mistakes students make, along with tips on how to avoid them:
| Common Mistake | How to Avoid It |
|---|---|
| Misreading the question | Always read questions carefully and underline important details before answering. |
| Incorrect unit conversions | Double-check unit conversions and ensure consistency in all calculations. |
| Not balancing equations properly | Take extra time to balance equations and verify the stoichiometric relationships. |
| Overlooking significant figures | Ensure all calculations and final answers reflect the correct number of significant figures. |
| Failing to show work | Write out all intermediate steps to ensure clarity and avoid simple errors. |
| Rushing through calculations | Take your time and check each step before finalizing your answer. |
By being mindful of these common mistakes, you can avoid unnecessary errors and approach problems with more confidence and precision. Practice regularly, and these habits will become second nature, improving both your speed and accuracy.
Formulas to Memorize Before the Exam
Mastering key formulas is essential for performing well on assessments. These mathematical relationships form the foundation for solving many problems and help simplify complex concepts. Familiarizing yourself with the most important equations will allow you to approach questions confidently and efficiently. Here, we highlight the key formulas you should have at your fingertips before stepping into the test environment.
Important Equations
- Ideal Gas Law: PV = nRT – Describes the relationship between pressure, volume, temperature, and the number of moles of gas.
- Molarity: M = mol/L – Defines the concentration of a solution in moles per liter.
- Heat Equation: q = mcΔT – Calculates the amount of heat energy transferred, where m is mass, c is specific heat capacity, and ΔT is the temperature change.
- Law of Conservation of Mass: m_reactants = m_products – States that mass is conserved in a chemical reaction.
Key Conversion Factors
- Avogadro’s Number: 6.022 x 10^23 – The number of particles (atoms, molecules, etc.) in one mole of a substance.
- Rydberg Constant: R = 1.097 x 10^7 m⁻¹ – Used in equations for hydrogen atom spectral lines.
- Specific Heat Capacity: c = J/(g°C) – The amount of heat required to raise the temperature of a gram of a substance by one degree Celsius.
These are just a few of the key formulas that you should commit to memory. Knowing these equations will save you time and improve your problem-solving abilities, allowing you to approach a wide range of questions with ease. Regular practice and familiarity with these formulas will make them second nature, ensuring you are well-prepared for any challenge.
Study Techniques for Better Retention
Effective study methods are crucial for ensuring long-term retention of material and understanding complex concepts. Rather than simply reviewing notes, it’s important to adopt strategies that reinforce memory and understanding. By engaging with the content actively and applying various techniques, you can enhance both recall and comprehension. This section outlines several approaches to maximize your learning potential.
Active Recall
Active recall involves testing yourself regularly on the material, instead of passively reading through your notes. This technique forces your brain to retrieve information, strengthening neural connections and making it easier to remember the material during assessments. You can use flashcards, practice questions, or write out what you’ve learned from memory.
Spaced Repetition
Spaced repetition is a method where you review material at increasing intervals over time. This technique takes advantage of the brain’s ability to retain information better when it’s revisited periodically. Using apps or flashcards that use spaced repetition algorithms can help you reinforce key concepts just before you’re about to forget them.
Additionally, breaking down study sessions into shorter, focused periods (also known as the Pomodoro technique) can help maintain high levels of focus and reduce mental fatigue. Regular breaks, combined with consistent review sessions, help solidify the information you’ve learned.
By incorporating these methods into your study routine, you can significantly improve your retention of complex concepts and be better prepared for any upcoming challenges.
Group Study vs Individual Review

When preparing for challenging assessments, students often face the decision of whether to study alone or in a group. Both approaches offer distinct advantages, but the choice largely depends on personal preferences and learning styles. In this section, we will compare the benefits of group study sessions with those of solitary study methods, helping you decide which might be more effective for your preparation.
Group study can foster a collaborative environment where students can share knowledge, discuss difficult concepts, and work through complex problems together. The exchange of ideas often leads to new insights and a deeper understanding of the material. Additionally, studying in a group can keep you motivated and accountable, as others can help you stay on track and offer support when needed.
On the other hand, individual study allows for a more personalized approach. You can focus entirely on areas where you need the most improvement, set your own pace, and minimize distractions. With this method, it’s easier to structure your sessions around your own strengths and weaknesses, allowing for more concentrated and independent learning.
Ultimately, both methods have their place in preparation. Combining the two–engaging in group study for collaborative learning and turning to solo review for deep, focused study–can often provide the most comprehensive approach to mastering the material.
Exam Day Preparation and Tips
The day of a major assessment can be both exciting and nerve-wracking. To perform at your best, it’s crucial to prepare both mentally and physically. Proper preparation on the day itself can significantly impact your performance and reduce unnecessary stress. In this section, we will cover essential steps to take on the day of your assessment to ensure you’re in the right mindset and ready to tackle any challenges that come your way.
Morning of the Assessment
- Get enough sleep: Aim for 7-8 hours of rest the night before to help improve focus and memory retention.
- Eat a balanced breakfast: Choose foods that will keep you energized, such as whole grains, protein, and fruit. Avoid heavy or sugary foods that may lead to an energy crash.
- Stay hydrated: Drink plenty of water in the morning to maintain optimal brain function.
- Review key concepts: Do a light review of essential topics or tricky areas, but avoid cramming. Trust the preparation you’ve already done.
Before You Start the Assessment
- Arrive early: Get to the location with plenty of time to spare so you can settle in and avoid any last-minute rush.
- Bring necessary materials: Make sure you have everything you need, such as identification, writing tools, a calculator, or any allowed reference materials.
- Calm your nerves: Take deep breaths or do simple relaxation exercises to calm any pre-assessment anxiety. Confidence is key.
With the right approach, you can ensure that you’re mentally prepared and physically ready to succeed. Taking these steps on the day of the assessment will help you perform your best under pressure.