
When encountering unfamiliar materials, the process of identifying their characteristics and properties is both a challenging and fascinating scientific task. With the right approach, a series of simple experiments can reveal the nature of these unknown substances. This section focuses on various methods and techniques that help in deciphering the unknown components, relying on observation, reaction, and analysis.
Experiments involving unmarked compounds offer a unique opportunity to apply critical thinking and scientific reasoning. By examining texture, solubility, reaction to heat, and other properties, it is possible to make informed conclusions. Understanding the behavior of substances under different conditions provides valuable insights into their composition and potential uses.
Effective identification relies on a systematic approach that combines scientific knowledge with hands-on experimentation. This guide will walk through essential steps, common challenges, and tips for successfully determining the composition of unknown materials, enhancing your problem-solving skills in the process.
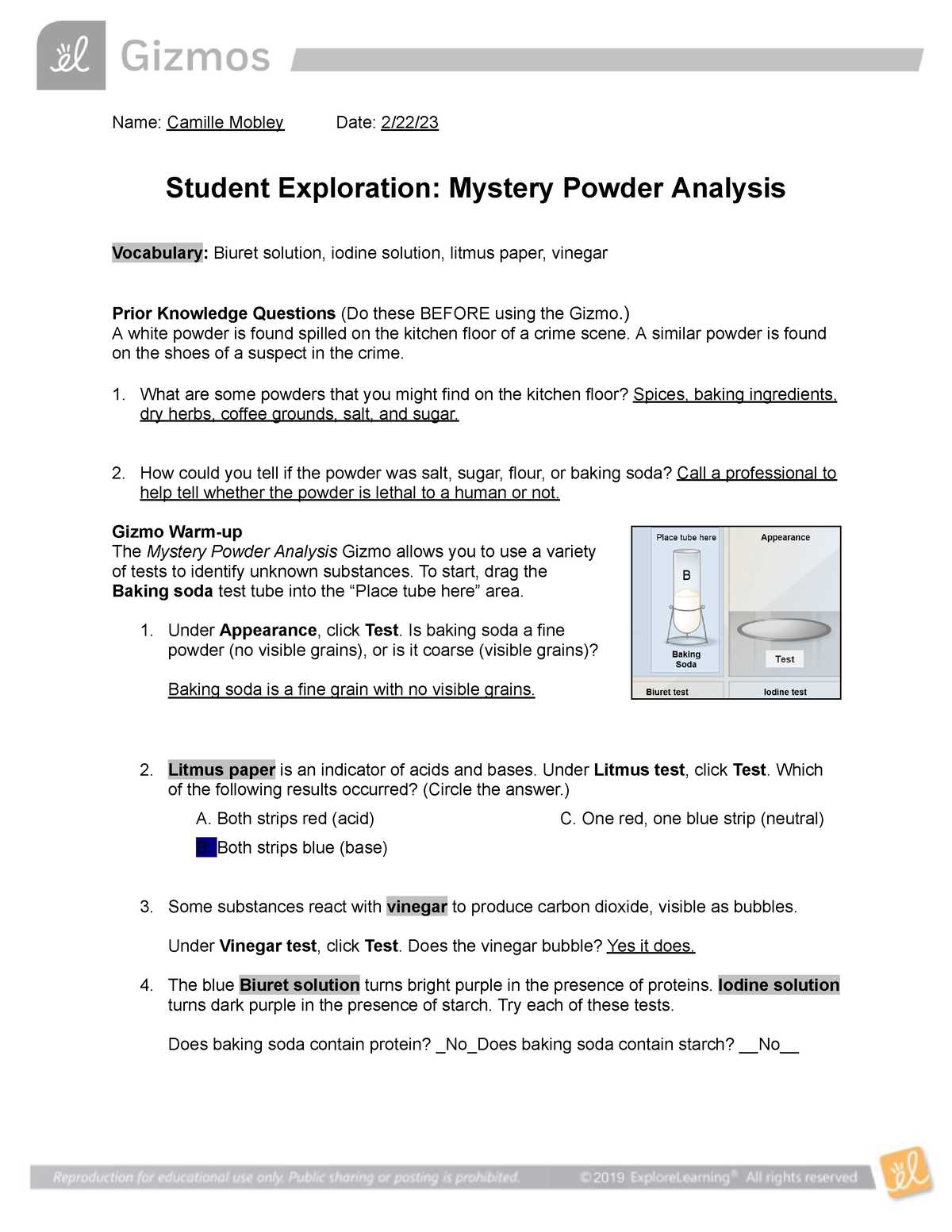
Mystery Powder Lab Answer Key
In any scientific investigation involving unidentified substances, determining their true nature requires a systematic and precise approach. By performing various tests, scientists can identify key characteristics that lead to the recognition of the material. This section provides essential insights and solutions for interpreting the results of such experiments, helping to connect observed reactions with the specific traits of the substances in question.
Through a series of simple yet effective techniques, each sample undergoes scrutiny to reveal its unique properties. Whether it’s examining changes in color, reaction to heat, or solubility in different solvents, these experiments provide a clear path to identification. The data gathered through each test serves as a clue, building the case for the identity of the substance.
The goal of this section is to clarify how each test works and guide the interpretation of the results. By following the correct procedures, even novice scientists can arrive at accurate conclusions regarding the materials being studied. Understanding the connection between each experiment and the final identification is crucial for successful analysis and learning.
Understanding the Mystery Powder Experiment
Scientific experiments involving unknown substances are designed to reveal their composition through observation and testing. These exercises challenge participants to apply their knowledge of chemistry and materials science to identify the nature of unfamiliar compounds. The focus lies in utilizing various methods, such as physical properties, chemical reactions, and solubility, to differentiate one substance from another.
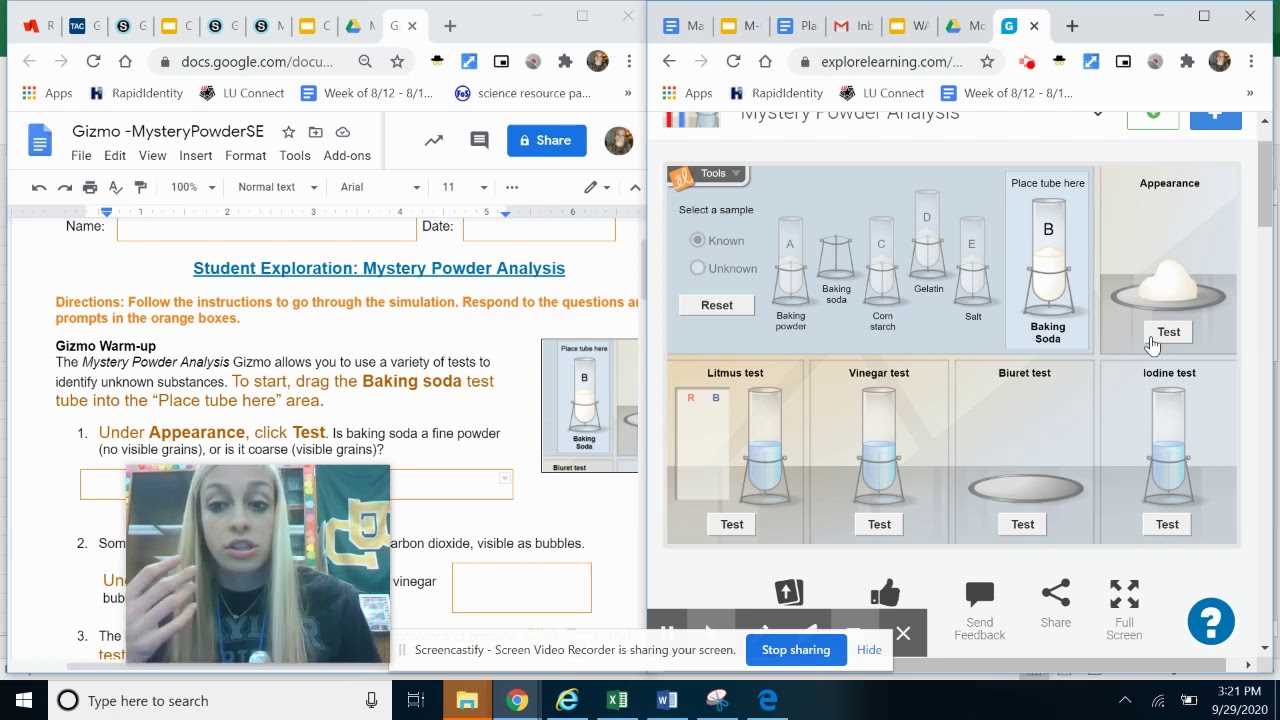
Identifying Key Properties
Each sample in the experiment exhibits specific characteristics that can provide valuable clues about its identity. Whether it’s a reaction to heat, its behavior in different solvents, or changes in texture, these features are essential in narrowing down the possibilities. Identifying the right tests and interpreting their results accurately is crucial for successful identification.
Applying Systematic Testing
To ensure reliable results, it’s important to follow a set procedure when conducting experiments. This allows for consistent observations and accurate conclusions. Understanding how to test for reactivity, solubility, and other key properties will guide the identification process, ensuring that each step contributes to revealing the substance’s true nature.
Key Techniques for Identifying Powders
To identify unknown substances, it is essential to use a combination of scientific methods that examine both physical and chemical properties. By employing these techniques, scientists can gather important clues that lead to accurate identification. Each method provides unique insights into the characteristics of the material, helping to eliminate possibilities and narrow down the options.
One of the fundamental techniques involves testing solubility. By observing how a sample dissolves in various liquids, it is possible to gain information about its chemical composition. Another effective approach is heat testing, where reactions to temperature changes reveal crucial details about the material’s stability and behavior. Other methods include observing the texture, color, and reactivity with common substances such as acids or bases.
These techniques, when combined, provide a comprehensive understanding of the substance in question. Properly interpreting the results of each test ensures a reliable process for identifying unknown materials with precision.
Scientific Methods in Powder Analysis
Analyzing an unknown substance requires a set of scientific techniques designed to uncover its fundamental characteristics. By applying systematic methods, scientists can accurately determine the material’s composition and behavior under different conditions. These methods rely on precise measurements and careful observation, allowing for the identification of key properties such as solubility, reactivity, and physical texture.
Reactivity Tests and Observations

One of the most effective ways to analyze an unknown material is through its reaction to certain chemicals or environmental changes. For example, testing how a substance interacts with acids, bases, or heat can provide valuable insights into its chemical structure. These reactions often produce visible changes such as color shifts, bubbling, or the formation of precipitates, which are crucial in identifying the compound.
Physical Properties and Their Importance
In addition to chemical reactions, understanding the physical characteristics of a sample is critical. Texture, color, and particle size are all important factors that can help narrow down the possibilities. Observing these traits can often lead to immediate clues, particularly when combined with other tests. By comparing these properties to known substances, a more accurate identification can be achieved.
How to Perform Simple Tests
Conducting basic experiments can be an effective way to identify the composition of an unknown substance. These tests rely on observing how the material reacts under various conditions, such as changes in temperature, interaction with liquids, or exposure to different chemicals. Simple tests can provide immediate clues that, when interpreted correctly, help pinpoint the substance’s nature.
Testing for Solubility
One of the first tests to perform is solubility. By dissolving a sample in water or another solvent, you can determine whether the material is soluble or insoluble. This is an important indicator of the substance’s composition, as different compounds exhibit distinct solubility characteristics. Carefully observe how the material interacts with the solvent, noting whether it dissolves completely or forms a suspension.
Heat Reaction Observations
Another straightforward method is testing how the substance reacts when exposed to heat. Heating the material can cause changes in its appearance, texture, or even its chemical structure. Pay attention to any changes such as melting, burning, or the release of gases. These reactions provide valuable information about the substance’s properties and help in identifying its nature.
What to Expect from the Lab Results
After conducting tests on an unknown substance, it’s important to carefully analyze the results to determine its identity. The outcomes of these experiments provide valuable clues about the material’s properties and help confirm hypotheses. Understanding what to look for in the results can significantly aid in identifying the compound with precision.
Interpreting Physical and Chemical Changes
The first step in analyzing the results is to evaluate any physical or chemical changes observed during testing. These changes can include color shifts, texture alterations, or reactions like bubbling or precipitation. Such transformations are often direct indicators of the substance’s composition. For example, a substance that reacts with acid to produce bubbles could be a carbonated compound.
Using Comparison to Known Substances
Once the reactions are observed, it’s helpful to compare the results to known substances with similar characteristics. This comparison can be made based on the chemical properties and physical traits of the substance, such as solubility or reaction to heat. The more tests you conduct, the closer you’ll get to identifying the material with confidence.
| Test Conducted | Expected Result | Possible Identification |
|---|---|---|
| Reaction with water | Forms a solution or precipitate | Salt or acid |
| Reaction to heat | Melts or burns | Organic or inorganic material |
| Solubility test | Dissolves in alcohol | Organic compound |
| Reaction with acid | Bubbling or color change | Carbonate or metal compound |
Common Substances in Mystery Powder Lab
In experiments that involve identifying unknown materials, certain substances are frequently encountered due to their common chemical properties and reactions. These substances can be easily identified through basic tests and observations. Understanding the characteristics of these common compounds is essential for recognizing them when they appear in experiments.
Common Substances and Their Properties
- Sodium chloride (NaCl) – A common salt, dissolves easily in water, forms a clear solution, and has no reaction to heat.
- Calcium carbonate (CaCO3) – Reacts with acids, producing bubbles of carbon dioxide, commonly found in chalk or limestone.
- Citric acid (C6H8O7) – Found in citrus fruits, soluble in water, and reacts with bases to form salts and water.
- Sugar (C12H22O11) – Soluble in water, often sweet-tasting, and does not react to heat but caramelizes when heated.
- Baking soda (NaHCO3) – Reacts with acids to produce carbon dioxide gas, used in cooking and cleaning.
Physical and Chemical Behavior
Each substance behaves differently under various conditions. For instance, when exposed to heat, some may melt, while others may decompose or emit gases. Observing these reactions helps narrow down the possibilities, especially when multiple tests are conducted in parallel. By understanding these common substances and their characteristics, accurate identification becomes much easier during experiments.
Interpreting the Color Changes in Tests
Color changes during testing can provide significant clues about the chemical composition and reactions of an unknown substance. These changes often indicate a chemical transformation, where the material interacts with other substances or undergoes a shift in its chemical state. Observing and interpreting these color shifts is a critical step in identifying the compound’s nature.
Common Color Reactions and Their Meanings
Various chemical reactions result in noticeable color changes that can help identify specific substances. For example, when exposed to acids, certain compounds may turn red, while others may shift to blue or green. Each color is indicative of particular chemical bonds or ions that are present in the material. Below are some common reactions and their interpretations:
| Test | Expected Color Change | Possible Substance |
|---|---|---|
| Reaction with litmus paper | Red (acidic) or blue (alkaline) | Acid or base |
| Reaction with iodine | Yellow to brown | Starch |
| Reaction with phenolphthalein | Colorless to pink | Base (e.g., sodium hydroxide) |
| Reaction with copper sulfate | Blue solution | Copper salts |
Factors Influencing Color Changes
It is important to note that various factors can influence the color changes observed during testing. Temperature, concentration of reagents, and the presence of other substances can all affect the intensity and type of color shift. Thus, careful control of experimental conditions is essential for accurate interpretation.
Using Chemical Reactions for Identification
Chemical reactions play a vital role in determining the identity of unknown substances. By observing how a material behaves when combined with certain reagents, scientists can deduce its composition. These reactions can cause visible changes such as color shifts, gas production, or the formation of precipitates, all of which provide clues to the nature of the substance. Understanding these reactions allows for more accurate identification and classification of unknown materials.
Common Chemical Reactions for Identification
Several chemical tests are commonly used to identify materials based on their reactions with specific reagents. These tests take advantage of well-known chemical properties to produce distinct results. Below are a few examples:
- Acid-Base Reactions: When a substance reacts with an acid or base, it can result in the release of gas, a color change, or a temperature shift. For example, a reaction with hydrochloric acid may release carbon dioxide from carbonates.
- Oxidation-Reduction Reactions: These reactions involve the transfer of electrons, often resulting in color changes or the formation of new compounds. A typical example is the reaction between copper and oxygen to form copper oxide, which has a distinct color.
- Precipitation Reactions: When two solutions are mixed, a solid precipitate may form, indicating the presence of certain ions or compounds. For example, adding silver nitrate to a chloride solution forms a white precipitate of silver chloride.
Using Reactions to Narrow Down Substances
After conducting chemical tests, it’s essential to interpret the results in light of known reactions and substances. For example, if a substance produces a yellow precipitate when mixed with lead nitrate, it may be identified as a halide. By systematically performing different tests, the list of possible substances can be narrowed down, leading to a more precise identification.
Factors Affecting Experiment Accuracy
Several factors can influence the accuracy and reliability of experimental results. These factors must be carefully controlled to ensure that the conclusions drawn from the experiment are valid. Variations in the environment, equipment, or method can lead to discrepancies, making it crucial to understand and account for these variables in the experimental process.
Key Factors Impacting Results
To achieve accurate and reproducible results, it’s important to identify and manage the various factors that can affect an experiment. Below are some of the most common elements that can influence the outcome:
- Temperature: Temperature fluctuations can affect chemical reactions, the solubility of substances, and the rate of reactions, leading to inconsistent results.
- Measurement Precision: The accuracy of instruments used to measure substances, liquids, or gases is critical. Small errors in measurement can significantly affect the outcome of an experiment.
- Contamination: The presence of impurities or foreign substances in reagents or equipment can alter the expected results and lead to misleading conclusions.
- Human Error: Mistakes in performing tests, such as incorrect timing or improper handling of chemicals, can introduce errors that affect the validity of the results.
- Reagent Purity: The quality and composition of reagents used in experiments play a key role. Impure or degraded reagents can result in incorrect reactions or unexpected outcomes.
Minimizing Errors and Maximizing Accuracy
To reduce errors and improve accuracy, it is essential to implement precise techniques and maintain consistency throughout the experiment. Proper calibration of instruments, careful measurement of reagents, and controlling environmental factors such as temperature are crucial steps. Additionally, repeating the experiment and cross-checking results can help identify and minimize discrepancies.
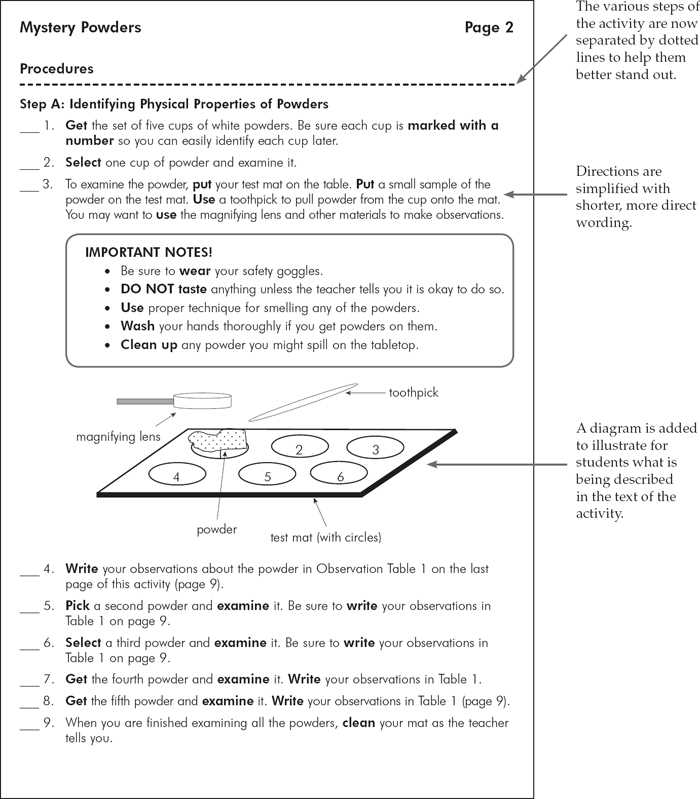
Identifying Powders Based on Texture
The texture of a substance can provide valuable clues in its identification process. By examining how a material feels, behaves, or interacts with other substances, it is possible to distinguish between different types of materials. Textural properties such as smoothness, grittiness, and clumpiness are often characteristic of certain compounds and can help narrow down the possibilities for identification.
When handling a sample, observing its tactile properties is one of the first steps in gathering useful information. The way the material responds to pressure or moisture, or whether it forms clumps or remains granular, can point to specific types of compounds.
| Texture | Possible Identification |
|---|---|
| Smooth, fine | Common in salts like sodium chloride or fine flour |
| Gritty, rough | Indicates materials like sand or some metal oxides |
| Clumpy, sticky | Possible organic materials or compounds like starch |
| Powdery, loose | Could indicate substances like talc or some fertilizers |
It is important to note that texture alone cannot conclusively identify a substance but should be considered in conjunction with other tests and observations. Combining textural analysis with chemical tests can increase the accuracy of identification and help form a clearer picture of the material in question.
How Temperature Affects Powder Reactions
Temperature plays a crucial role in the way substances react to one another. It can influence the speed, intensity, and type of reactions that occur. When heat is applied to certain materials, it can cause them to react more rapidly, change state, or even decompose. Understanding how temperature affects these reactions is essential for identifying and analyzing materials effectively.
In many experiments, temperature variations can provide key insights into the properties of the substance being tested. Higher temperatures often increase the rate of chemical reactions by providing more energy to the molecules involved. Conversely, lower temperatures may slow reactions down or prevent certain processes from occurring altogether. These effects are vital in distinguishing between different materials based on how they react to temperature changes.
Effects of High Temperature
When exposed to elevated temperatures, certain substances may undergo significant changes, including:
- Decomposition: Some materials break down into simpler substances when heated, making it easier to identify their components.
- Increased Solubility: Heat can increase the solubility of some substances, causing them to dissolve in solvents more easily.
- Color Changes: Many reactions are accompanied by color shifts when exposed to high temperatures, which can be an indicator of specific chemical processes.
Effects of Low Temperature
On the other hand, cooling a substance may cause it to behave differently. Low temperatures can result in:
- Slower Reactions: Chemical reactions generally proceed more slowly at lower temperatures, making it harder to observe rapid changes.
- Freezing: Some materials may freeze or solidify at low temperatures, providing clues about their physical properties.
- Reduced Reactivity: In some cases, lowering the temperature can make substances less reactive or prevent reactions from occurring altogether.
By carefully controlling and observing the effects of temperature, it is possible to draw meaningful conclusions about the identity and behavior of substances in a controlled environment.
Role of Solubility in Identification
Solubility is an essential property when identifying substances. The ability of a material to dissolve in a solvent can provide significant clues about its chemical composition. Solubility tests often involve mixing the sample with water or other solvents to observe how it behaves. Some materials dissolve completely, while others may only dissolve partially or not at all. These reactions can help narrow down the identity of the substance.
Understanding solubility is critical because it often correlates with other physical and chemical properties, such as polarity or molecular structure. By performing solubility tests, one can distinguish between various types of materials, as many compounds have characteristic solubility profiles. These tests can also be useful in confirming the results of other experimental procedures.
Solubility in Water
Water solubility is one of the most common and useful tests in substance identification. Some materials dissolve easily in water, while others remain insoluble. Observing the behavior of a sample in water can help identify substances such as:
- Sodium chloride: A common salt that dissolves easily in water.
- Sugar: Another substance that dissolves readily, producing a clear solution.
- Sand: Insoluble in water and remains as a solid.
Solubility in Organic Solvents
In addition to water, many substances dissolve in organic solvents such as ethanol, acetone, or oil. Testing solubility in different solvents can reveal further insights into the nature of a substance. For example:
- Oil-based compounds: Many organic compounds dissolve more readily in oils than in water.
- Acetone-soluble materials: Substances like some plastics and oils may dissolve in acetone but remain undissolved in water.
By systematically testing the solubility of a sample in various solvents, it becomes easier to classify and identify the unknown substance based on its solubility behavior.
Step-by-Step Guide for Lab Analysis
Conducting a thorough analysis of an unknown substance involves a structured approach to ensure accurate results. Each step in the process helps narrow down the possibilities and reveals more about the composition of the sample. A systematic procedure is critical for obtaining reliable data and minimizing errors during the experiment. In this guide, we will break down the process into clear, manageable steps to help you efficiently analyze a sample and interpret the results.
Preparing the Sample
The first step in any analysis is proper preparation. Before performing any tests, it is crucial to ensure the sample is handled safely and appropriately. Begin by measuring the substance to ensure you have enough material for the tests. If necessary, divide the sample into smaller portions to use in different tests. Always wear appropriate safety equipment such as gloves and goggles, and work in a well-ventilated area or fume hood to avoid exposure to harmful fumes.
Performing Tests and Observations
Once the sample is prepared, begin with basic tests to observe its physical properties. This might include tests such as:
- Solubility Tests: Determine how the substance behaves when mixed with water or organic solvents. Record whether it dissolves or remains intact.
- Color and Texture Analysis: Observe any color changes or texture variations when the sample is exposed to different conditions.
- Heat Reaction: Expose the sample to heat and observe any changes in its physical state or the release of gases.
Throughout each test, document your observations carefully. The physical characteristics and reactions will provide important clues to the identity of the sample.
Interpreting Results
After completing the tests, analyze the results to form a conclusion about the substance. Compare your observations to known data on similar materials. For example, the solubility, color changes, and reactions to heat may suggest certain properties like the presence of salts, organic compounds, or metals. It is important to cross-reference your findings with scientific literature or databases for a more accurate identification.
By following these steps carefully, you can increase the accuracy of your analysis and make well-informed conclusions about the unknown sample.
Challenges in Identifying Unknown Substances
Identifying an unknown substance can be a complex and sometimes frustrating process. There are numerous factors that can complicate the analysis, from the physical properties of the material to the limitations of the testing methods used. Each sample may exhibit unique characteristics that require careful observation and interpretation. In this section, we will explore some of the key challenges faced during the identification process and the ways to overcome them.
Similarities Between Substances
One of the most significant challenges in identifying an unknown material is the potential for similar properties between different substances. Many compounds share common characteristics, such as color, texture, or solubility. For example, salt and sugar both appear as white crystalline solids and dissolve in water, making it difficult to distinguish between them without further testing. It is crucial to perform a range of tests to rule out similar substances and narrow down the possibilities.
Interference from Contaminants
Another challenge arises when a sample is contaminated with other materials. Even trace amounts of impurities can alter the results of tests and lead to inaccurate conclusions. Contaminants may cause unexpected reactions or affect the solubility, color, or texture of the sample. To minimize this issue, it is important to ensure proper sample collection and handling techniques, as well as to use clean equipment and controlled testing conditions.
Limited Testing Methods
Sometimes, the tests available may not provide enough information to definitively identify a substance. Certain compounds may not react in ways that are easily observable or distinguishable under standard experimental conditions. In such cases, advanced testing methods, such as spectrometry or chromatography, might be necessary. However, these techniques are often costly and may not be accessible in all environments, further complicating the identification process.
Despite these challenges, with careful attention to detail and the use of a variety of testing methods, it is often possible to identify the unknown substance accurately. Patience, thoroughness, and an understanding of the limitations of each technique are essential in overcoming the difficulties associated with substance identification.
Safety Precautions for Substance Testing
When conducting tests on unknown materials, it is essential to prioritize safety to prevent accidents and ensure accurate results. Various substances may behave unpredictably, and proper precautions should be in place to avoid harmful reactions or exposure to hazardous chemicals. This section covers key safety guidelines and procedures to follow when handling and analyzing unfamiliar substances in a controlled environment.
Protective Equipment
Before beginning any testing, ensure that all necessary protective gear is worn. This helps minimize the risk of injury or exposure to dangerous chemicals. Key protective equipment includes:
- Safety goggles: To protect eyes from splashes, fumes, or particles.
- Gloves: To prevent direct contact with substances that could irritate the skin or cause chemical burns.
- Lab coat: To protect clothing and skin from spills or splashes.
- Face mask or respirator: To reduce inhalation of potentially toxic fumes or fine particles.
Handling and Disposal Procedures
Proper handling and disposal of materials are crucial to maintaining safety during substance testing. Follow these guidelines to avoid contamination or exposure:
- Label all substances: Clearly mark all containers and materials to ensure they are easily identifiable.
- Minimize exposure: Work in a well-ventilated area, ideally under a fume hood, to avoid inhaling fumes or dust.
- Dispose of waste properly: Follow all local regulations and safety protocols for disposing of chemicals or other testing materials.
- Avoid mixing unknown substances: Unless a reaction is part of the experiment, never combine substances without knowing how they will interact.
Precautions During Reactions
Certain reactions can be volatile or produce harmful byproducts. To ensure safety during chemical reactions:
- Monitor reactions closely: Always observe the material closely during testing to detect any changes or unexpected reactions.
- Use appropriate containment: Conduct reactions in containers designed for the specific test to contain any spills or eruptions.
- Have emergency procedures in place: Be prepared for potential accidents, such as spills or fires, and know how to respond quickly. Keep emergency equipment, such as eyewash stations and fire extinguishers, readily available.
By following these safety precautions, you can create a safe environment for testing and ensure that all procedures are carried out effectively and responsibly. Always be mindful of the risks associated with handling unknown substances, and never hesitate to seek assistance if needed.
Common Mistakes to Avoid in Experiments
When conducting experiments, especially those involving unfamiliar substances, there are several common pitfalls that can lead to inaccurate results or even safety hazards. Avoiding these mistakes is crucial for obtaining reliable data and ensuring the safety of everyone involved. This section outlines key errors to watch out for and provides tips for conducting tests more effectively.
One of the most frequent mistakes is not carefully reading and understanding the instructions or procedure before starting. Incomplete knowledge can lead to improper handling of materials or misinterpretation of test results. It is essential to fully understand the steps, precautions, and expected outcomes before beginning any experiment.
1. Incorrect Measurements
Accurate measurements are fundamental to any experiment. Using the wrong quantities of substances or equipment can lead to faulty conclusions. Always double-check measurements, and use the appropriate tools to ensure precision. Some specific errors include:
- Not using calibrated equipment: Ensure that scales, thermometers, and other measuring instruments are properly calibrated to avoid discrepancies.
- Misreading instruments: Always check readings at eye level to prevent parallax errors, and ensure that measurements are taken at the correct phase of the process.
- Failure to account for environmental factors: Temperature, humidity, and atmospheric pressure can affect readings, so it is important to control or account for these variables when measuring.
2. Poor Record Keeping
Failing to record observations and results meticulously can lead to confusion or errors in analysis. Consistent and accurate documentation allows for easier review and comparison of results. Common issues include:
- Skipping or neglecting observations: Every detail counts, from visual changes to reactions or temperature shifts. Skipping any observation can affect the interpretation of results.
- Inconsistent note-taking: Record data in a clear and systematic manner. Use consistent units, terms, and formats to avoid misunderstandings or mix-ups later.
3. Rushing the Process
Speed is often a temptation, but hasty actions can lead to errors that might be costly or dangerous. It is essential to take the time necessary to follow all procedures properly and allow reactions or processes to proceed at their own pace. Rushing can cause:
- Overlooking safety measures: In the interest of time, safety precautions may be skipped, which can lead to accidents or exposure to harmful chemicals.
- Inaccurate timing: Many experiments require precise timing. Rushing through timing or skipping stages can result in incorrect outcomes.
By avoiding these common mistakes, the integrity of the experiment will be preserved, and reliable results can be achieved. Always take your time, be precise, and adhere to safety protocols to ensure the best outcomes.
Final Thoughts on Powder Identification
Identifying unknown substances is a challenging yet rewarding task that requires careful attention to detail and a solid understanding of various testing techniques. Whether the goal is to determine the composition of a material or to assess its properties, a structured approach is crucial for obtaining reliable results. Throughout the process, it is essential to maintain a thorough and methodical approach, as small errors can lead to misidentification or inaccurate conclusions.
While many methods exist for identifying unknown materials, each technique has its limitations and advantages. The key to success lies in combining multiple approaches to cross-check results and increase accuracy. This process involves analyzing the texture, solubility, reactions to various chemicals, and temperature sensitivity of the substance, among other factors. By understanding the strengths and weaknesses of each method, a more complete and accurate identification can be achieved.
1. Importance of Systematic Testing
A methodical approach is fundamental to successful identification. Rushing through tests or skipping certain procedures can lead to incomplete or incorrect results. Some essential steps to keep in mind include:
- Consistent observations: Always document changes thoroughly and consistently throughout the experiment.
- Repetition of tests: Perform tests multiple times to verify results and account for any inconsistencies.
- Cross-checking results: Use different methods to verify your conclusions and ensure accuracy.
2. Emphasizing Safety and Precision
Safety should always be a priority during any experiment. When working with unknown materials, it’s essential to take precautions to avoid potential hazards. Additionally, precision in measurements and observations is critical to obtaining valid results. To improve both safety and accuracy, remember the following:
- Wear appropriate protective gear: Gloves, goggles, and lab coats can prevent accidents and minimize exposure to harmful substances.
- Follow established protocols: Stick to the guidelines for each test to avoid mishandling or incorrect procedures.
- Record everything: Keeping detailed records of your observations helps maintain clarity and ensures that future steps can be accurately followed.
In conclusion, substance identification is a complex process that requires a balance of technical knowledge, attention to detail, and safety awareness. By using systematic testing methods, cross-checking results, and prioritizing safety, accurate identification can be achieved. This process not only aids in understanding the substance in question but also deepens one’s overall scientific knowledge and problem-solving skills.