Exploring the fundamental components that make up all substances provides insight into the core principles of chemistry and physics. By examining how tiny particles interact and combine, we gain a deeper appreciation for the physical world around us. This journey into the microcosm helps explain everything from the formation of elements to complex chemical reactions.
Learning about particles such as electrons, protons, and neutrons is essential for understanding the nature of matter. Each plays a unique role in shaping the properties of different substances. The arrangement and behavior of these particles determine the characteristics of various elements and compounds.
In this section, we will guide you through key concepts related to the components that form the foundation of all substances. Understanding these concepts is crucial for anyone studying the natural world, as they form the basis for further exploration in science and technology.
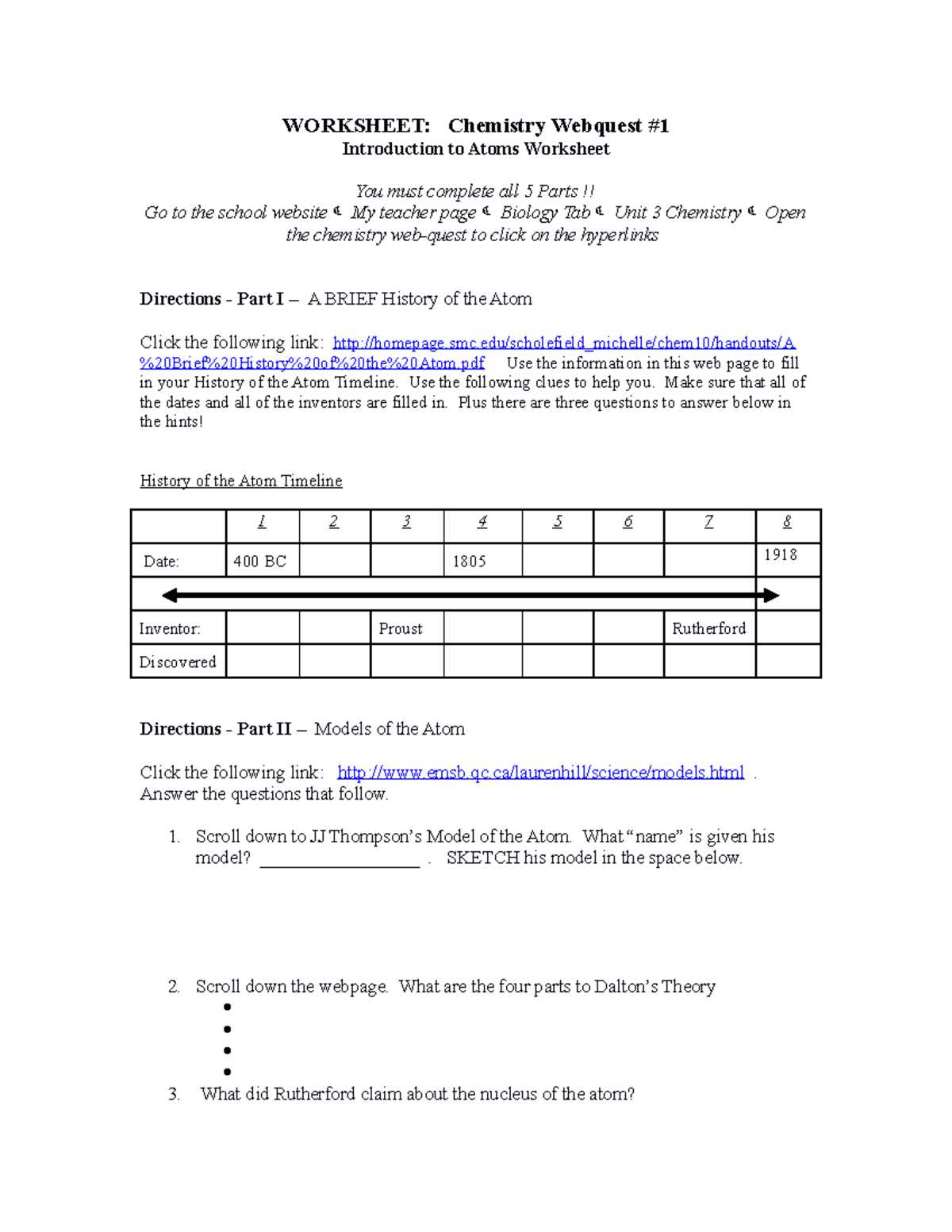
Understanding the Basics of Atomic Theory
The foundation of modern science lies in understanding how the smallest particles of matter behave and interact. By studying these minute building blocks, we unlock the secrets that govern everything from chemical reactions to the physical properties of substances. The idea that matter is composed of indivisible units has evolved over centuries, leading to the development of a comprehensive theory that explains the behavior of elements and compounds.
Early models of matter proposed by ancient philosophers laid the groundwork for this theory, although it wasn’t until the 19th and 20th centuries that scientific discoveries began to clarify the true nature of these particles. Through experimentation and observation, scientists began to realize that matter is not continuous but made up of discrete particles that follow specific rules of interaction.
One of the key principles in understanding these particles is recognizing that their properties–such as mass, charge, and energy–determine how they combine and react with each other. These fundamental aspects form the core of the theory, offering a framework for explaining everything from the simplest chemical bonds to complex molecular structures.
Key Concepts in Atomic Structure
At the heart of matter lies a set of fundamental principles that govern how particles interact and form the elements we encounter every day. Understanding these key ideas allows us to explain a vast range of natural phenomena, from chemical reactions to the physical properties of substances. These concepts are essential for grasping how matter behaves on a microscopic scale and how it influences the world around us.
One of the most important concepts is the idea that matter is made up of tiny, indivisible particles, each possessing specific properties that determine how they combine and interact. These particles are composed of smaller units, each with its own characteristics, such as mass and charge. The way these units arrange themselves and the forces acting between them play a crucial role in shaping the behavior of different materials.
Another critical aspect involves understanding how the properties of these units–such as their charge, mass, and energy–affect the behavior of elements. The arrangement of these units in specific patterns gives rise to different chemical and physical properties. By examining these key concepts, we can begin to make sense of the complexity of matter and predict how substances will react under various conditions.
Electron Configuration and Its Significance
The arrangement of particles within the smallest units of matter is key to understanding their chemical behavior and interactions. The way these units are distributed across different energy levels dictates how they form bonds and react with other elements. This distribution is often referred to as an element’s “electron configuration,” and it plays a crucial role in determining the properties of substances, including their reactivity, stability, and bonding characteristics.
Understanding Electron Placement
Each unit is surrounded by particles that occupy specific regions around the nucleus, called energy levels or shells. These regions can hold a certain number of particles, and the way they fill up determines how the unit behaves in chemical reactions. The arrangement follows a set of rules that explain why some elements are more reactive than others, why certain substances form particular types of bonds, and how these bonds impact the overall stability of molecules.
Importance in Chemical Behavior
The electron configuration of an element influences its ability to share, gain, or lose particles when interacting with other elements. This directly impacts the type of chemical bonds formed–whether ionic, covalent, or metallic–and explains why certain elements readily form compounds, while others remain inert. Understanding this concept allows chemists to predict the behavior of elements in various reactions and to design new materials with specific properties.
| Energy Level | Electrons | Example Element |
|---|---|---|
| 1 | 2 | Hydrogen |
| 2 | 8 | Oxygen |
| 3 | 18 | Argon |
Protons Neutrons and Electrons Explained
The building blocks of matter are composed of three fundamental types of particles that each contribute unique properties to the overall behavior of elements. These particles–protons, neutrons, and electrons–are the key players in determining how substances interact and how they are organized at a molecular level. Understanding the roles of these particles is crucial for exploring the properties of elements and their behavior in various reactions.
Protons and Their Role
Protons are positively charged particles found in the nucleus of an atom. The number of protons in an element’s nucleus defines its identity and places it on the periodic table. This is known as the atomic number. The positive charge of protons helps balance the negative charge of electrons, providing overall stability to the atom. Protons also play a key role in determining the chemical behavior of elements, as their number influences the bonding and reactivity of atoms.
Neutrons and Their Contribution
Neutrons, located alongside protons in the nucleus, are neutral particles that have no charge. Although they do not directly affect the chemical properties of an element, they are essential for stabilizing the nucleus. The number of neutrons in an atom can vary, leading to different forms of the same element, called isotopes. Isotopes of an element may have different physical properties but share the same chemical characteristics.
Electrons, on the other hand, are negatively charged particles that orbit the nucleus in various energy levels. Their distribution determines how atoms interact with other atoms and form chemical bonds. Electrons are the driving force behind the reactivity of elements, with atoms often seeking to gain, lose, or share electrons to achieve greater stability. The arrangement of electrons in shells and energy levels influences how elements combine and react in different conditions.
The Role of Atomic Models in Science
Throughout history, scientists have developed models to help explain the behavior of the smallest units of matter. These models are essential tools that provide a visual and conceptual framework for understanding complex ideas about how particles interact, combine, and behave. Without these models, much of modern chemistry and physics would remain abstract and difficult to grasp. Over time, atomic models have evolved as our understanding of the universe deepened, reflecting advances in scientific theory and experimental techniques.
Key Models in Scientific History
Various models have been proposed to describe the behavior of matter at the microscopic level. Each model has played a significant role in advancing our understanding of chemical reactions, energy transfer, and the nature of substances. Some of the most important models include:
- Dalton’s Model: The earliest conceptualization of matter, suggesting that elements are made up of indivisible particles called atoms.
- Thomson’s Model: Introduced the idea of electrons embedded in a “positive soup,” which led to the discovery of the electron.
- Rutherford’s Model: Proposed the existence of a dense nucleus, around which electrons orbit, based on experiments with gold foil.
- Bohr’s Model: Suggested that electrons orbit the nucleus in specific energy levels, explaining atomic spectra and stability.
- Quantum Mechanical Model: The modern model, which describes electron behavior using probabilistic distributions rather than fixed orbits.
Why Models Matter in Science
Models play a critical role in science by helping to simplify and clarify complex phenomena. They allow scientists to make predictions, design experiments, and visualize processes that are too small or too fast to observe directly. Furthermore, they provide a framework for testing new ideas and theories. As our understanding of the physical world advances, models are refined, expanded, or replaced to incorporate new data, making them dynamic and essential tools in scientific progress.
In short, atomic models are invaluable for explaining the nature of matter, guiding research, and pushing the boundaries of what we know about the universe.
How Atomic Number Defines Elements
The identity of an element is determined by the number of a specific type of particle present in its nucleus. This number plays a crucial role in defining an element’s properties and its place in the periodic table. It not only distinguishes one element from another but also governs how elements interact in chemical reactions, how they bond with other atoms, and what kind of compounds they form.
The number of protons in an atom’s nucleus is referred to as the atomic number. This value is unique to each element and dictates its position on the periodic table. As the atomic number increases, elements exhibit different characteristics, such as changes in their physical properties, reactivity, and the types of bonds they can form. The atomic number, therefore, directly influences the behavior and classification of elements.
| Element | Atomic Number | Symbol |
|---|---|---|
| Hydrogen | 1 | H |
| Oxygen | 8 | O |
| Carbon | 6 | C |
| Iron | 26 | Fe |
| Gold | 79 | Au |
Each element’s unique atomic number determines its chemical behavior and how it interacts with other substances. By understanding the atomic number, scientists can predict an element’s properties, including its stability, reactivity, and the types of compounds it is likely to form. It is the foundation of the periodic law, which states that elements with similar properties occur at regular intervals when arranged by increasing atomic number.
Neutron’s Role in Atomic Stability
The stability of an element depends on the balance between various particles within its nucleus. While the positive charge of protons plays a significant role in determining an element’s identity, another particle, the neutron, is crucial for maintaining the overall stability of the nucleus. Neutrons, which have no charge, help to hold the nucleus together by providing an essential force that counteracts the repulsive force between positively charged protons.
Neutrons and Nuclear Stability act as a stabilizing force within the nucleus. The balance between neutrons and protons is vital for preventing the nucleus from breaking apart. If the number of neutrons is too low or too high in relation to protons, the nucleus may become unstable, leading to radioactive decay or other forms of nuclear transformation. This delicate balance ensures that elements remain stable under normal conditions and can exist for long periods of time.
Neutron variations in elements can lead to the formation of isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are unstable and undergo radioactive decay to achieve a more stable configuration. Understanding the role of neutrons helps explain why certain elements can exist in multiple forms and how they behave in various environments.
Atomic Mass and Isotopes Overview
The mass of an element plays a critical role in understanding its behavior and characteristics. It is a combination of the mass of the particles in the nucleus–protons and neutrons–that defines how heavy an atom is. While all atoms of an element have the same number of protons, the number of neutrons can vary, leading to different forms of the same element, known as isotopes. This variation in neutrons directly influences the overall mass of the atom.
Atomic mass refers to the average mass of an element’s atoms, taking into account the different isotopes and their relative abundances in nature. It is often expressed in atomic mass units (amu). Since isotopes of an element have the same number of protons but different numbers of neutrons, their masses vary slightly. The more abundant isotopes contribute more to the average atomic mass, which is why the atomic mass listed on the periodic table is usually not a whole number.
Isotopes are versions of the same element that differ in the number of neutrons in their nuclei. While isotopes of an element exhibit nearly identical chemical properties, their physical properties–such as mass and stability–can differ. Some isotopes are stable, while others may be unstable and decay over time, emitting radiation. These radioactive isotopes are used in various applications, including medical imaging and dating archaeological samples.
Energy Levels and Orbitals Demystified
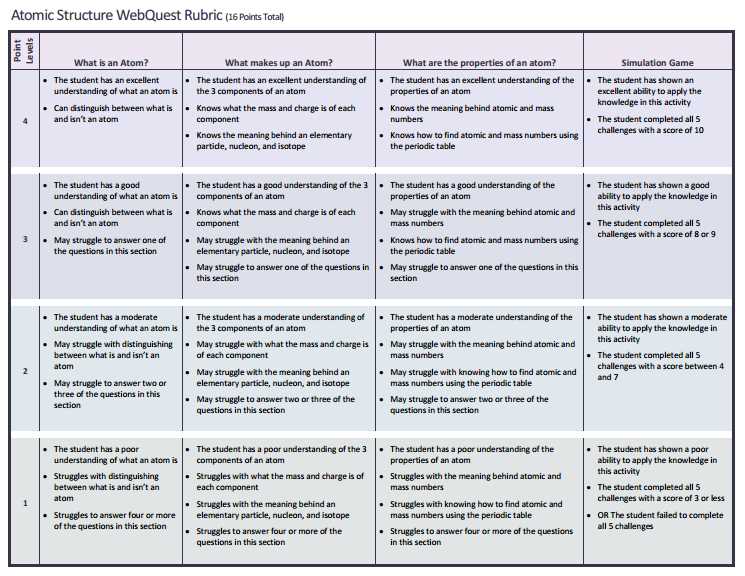
Understanding how particles behave within an atom is fundamental to grasping many concepts in chemistry and physics. At the heart of this behavior is the concept of energy levels, which define the regions around the nucleus where particles, such as electrons, are likely to be found. These levels are not just random distances from the nucleus but represent distinct energy states that electrons can occupy. Within each energy level, there are further subdivisions, called orbitals, that describe the specific locations or patterns where electrons are most likely to be found.
Energy levels are often described as “shells,” with each shell capable of holding a specific number of electrons. The farther an energy level is from the nucleus, the higher its energy and the greater the number of electrons it can hold. Electrons tend to occupy the lowest available energy level first, moving to higher levels only when the lower ones are filled. The number of energy levels in an atom depends on the element and its position in the periodic table.
Within each energy level, electrons occupy orbitals, which are regions of space where there is a high probability of finding an electron. Orbitals come in different shapes, such as spherical or dumbbell-shaped, and are designated by the letters s, p, d, and f. These orbitals define the distribution of electron density around the nucleus and determine how atoms interact with each other. The arrangement of electrons in these orbitals influences the chemical properties of elements, including their reactivity and bonding behavior.
Identifying Atomic Structure Through Webquest

Exploring the arrangement and behavior of particles within an element is an essential part of understanding the fundamentals of matter. Interactive learning tools, such as online explorations, allow students to engage with concepts in a dynamic way. Through guided exercises, learners can uncover key details about how particles like protons, neutrons, and electrons are organized and interact within an atom. These activities provide an opportunity to visualize concepts that can be challenging to grasp through textbooks alone.
In these interactive activities, students can examine how different elements vary in terms of their internal composition and how this influences their properties. By engaging in virtual simulations, users can manipulate variables such as particle count, energy levels, and electron arrangements, gaining a deeper understanding of how these factors affect an element’s behavior. Hands-on learning in this format enhances comprehension and retention, allowing for a more intuitive grasp of the complex concepts involved.
Learning through digital tools not only simplifies the process of visualizing the subatomic world, but it also supports learners in developing critical thinking and problem-solving skills. This method of discovery encourages students to experiment with different scenarios, observe the outcomes, and better understand the principles governing the behavior of matter on the smallest scale. Engaging with these resources makes abstract ideas more tangible, bridging the gap between theory and practical application in science.
Common Questions in Atomic Structure Webquest
When exploring the fundamental building blocks of matter, many common questions arise regarding how particles are organized within an element and how they interact. These inquiries often center on the nature of subatomic particles, their arrangement, and how their properties influence the behavior of elements. Through interactive learning experiences, students can gain clarity on these concepts by posing and answering key questions about matter’s composition.
One common question that arises is how the number of protons in the nucleus defines the identity of an element. This leads to further discussion on how electrons and neutrons contribute to the overall stability and behavior of atoms. Another frequent inquiry focuses on the relationship between the number of particles in an atom and its chemical properties, such as reactivity and bonding tendencies. Exploring these questions through hands-on digital tools helps reinforce understanding and provides a more comprehensive view of the topic.
Additionally, students often ask how variations in subatomic particles, like isotopes, affect the properties of elements. These variations can lead to different forms of the same element with distinct physical and chemical characteristics. Addressing these types of questions not only clarifies complex concepts but also encourages critical thinking and problem-solving as learners explore the subatomic world and its impact on macroscopic matter.
Solving Problems in Atomic Theory
Understanding the behavior of subatomic particles and how they interact is essential for solving problems related to the composition of matter. Many challenges arise when attempting to calculate various properties of elements, such as their mass, charge, or the distribution of particles within them. These problems often require a solid grasp of the underlying principles of particle behavior and how these principles can be applied to different scenarios.
One common approach to solving such problems is to use mathematical formulas and models to predict the properties of atoms. For example, calculating the number of electrons in a neutral atom, determining the charge of an ion, or predicting the number of isotopes involves understanding key relationships between protons, neutrons, and electrons. Here are some of the typical steps involved in solving problems in this area:
- Identify the known values: Start by gathering information such as the element’s atomic number, mass number, or number of protons.
- Use the periodic table: The periodic table provides crucial information on the number of protons and neutrons for each element, helping solve for unknown values.
- Apply relevant formulas: Depending on the problem, use specific equations for calculating electron configuration, ion charge, or isotope distribution.
- Double-check calculations: Ensure that calculations are consistent and the results align with known scientific principles.
Through solving these problems, students not only reinforce their understanding of fundamental principles but also improve their ability to apply theoretical knowledge to practical situations. By continuously tackling different scenarios, learners can develop the analytical skills needed to excel in the study of matter and its composition.
How to Interpret Atomic Diagrams
Visual representations of matter at the particle level can often appear complex, but understanding how to read these diagrams is crucial for grasping the behavior of elements and their components. Diagrams that depict the arrangement of subatomic particles provide a simplified view of an atom’s internal structure. These illustrations help convey information about the number and arrangement of protons, neutrons, and electrons, offering insight into the properties and behavior of different elements.
Understanding the Key Elements of Diagrams
When interpreting a diagram, it’s essential to identify the key features, such as:
- Nucleus: This is the central region of the atom, often depicted as a dense cluster of protons and neutrons.
- Electron Shells: These are the concentric rings around the nucleus, each representing a different energy level or orbit for the electrons.
- Electrons: Typically shown as particles revolving around the nucleus, their arrangement is important for understanding chemical behavior and reactivity.
Analyzing Different Types of Diagrams
Different types of diagrams can be used to represent atoms in varying degrees of complexity. Some diagrams show only the nucleus and electron shells, while others may include additional details, such as the relative energy levels of electrons or the ionization states of elements. To correctly interpret these representations, one must consider:
- Element Identity: The number of protons in the nucleus determines the element’s identity and its position on the periodic table.
- Electron Configuration: The arrangement of electrons in shells influences the chemical properties of the element and its ability to bond with others.
By understanding how to interpret these diagrams, students and learners can better grasp the concepts of matter and its behavior. This skill is essential for exploring complex topics like chemical bonding, reactions, and the nature of elements in different states.
Atomic Structure and Chemical Reactions
The behavior of matter and its transformation during chemical reactions is closely tied to the arrangement and interaction of its fundamental particles. Understanding how these particles–protons, neutrons, and electrons–play a role in reactions can provide valuable insight into how substances combine, break apart, or change form. The way in which atoms bond with one another or rearrange during reactions is a direct result of the behavior of electrons in outer energy levels or shells.
Electron Behavior and Chemical Bonds
The key to chemical reactions lies in the interactions between the outermost electrons of atoms. These electrons, found in the highest energy levels, are involved in forming chemical bonds. The nature of these bonds–whether covalent, ionic, or metallic–determines how atoms combine and the resulting properties of the compounds they form.
- Covalent Bonds: Occur when atoms share electrons to fill their outermost electron shells, creating molecules.
- Ionic Bonds: Form when one atom transfers electrons to another, resulting in charged ions that are attracted to each other.
- Metallic Bonds: Involve the sharing of free electrons among a lattice of metal atoms, giving metals their characteristic conductivity and malleability.
How Reactions Are Affected by Particle Interaction
During chemical reactions, the bonds between atoms are either broken or formed. These processes depend on the energy involved in the movement and interaction of electrons. The rate and type of reaction are influenced by factors such as temperature, pressure, and the presence of catalysts, which can affect how quickly particles collide and form new bonds.
- Reactivity: Atoms with more reactive electrons–especially those with incomplete outer shells–tend to form bonds more easily.
- Bond Energy: The energy required to break or form bonds determines the overall energy change in a chemical reaction, influencing whether the reaction is exothermic or endothermic.
By understanding how the behavior of electrons governs chemical reactions, scientists can predict how different substances will interact under various conditions, leading to breakthroughs in fields like material science, medicine, and energy production.
Resources for Mastering Atomic Structure
Grasping the principles behind the composition of matter requires access to various resources that can simplify complex concepts and enhance understanding. Whether you’re just beginning to explore the behavior of particles or seeking to deepen your knowledge of how matter interacts at the smallest levels, there are many tools available to support learning. From textbooks and online courses to interactive simulations, these resources offer diverse approaches to mastering the subject.
Books and Textbooks
Traditional textbooks remain one of the most comprehensive sources for in-depth knowledge on the topic. Many textbooks provide structured explanations, visual aids, and practice problems that help reinforce key concepts. Some notable textbooks include:
- “Chemistry: The Central Science” by Brown, LeMay, and Bursten: A widely used textbook that provides clear explanations and a solid foundation in the fundamentals of chemistry.
- “Principles of Chemistry” by McMurry and Fay: This book offers detailed sections on the behavior of matter and the forces at play within atoms.
Online Courses and Tutorials
Online courses allow learners to study at their own pace, offering flexibility and a variety of learning styles, from videos to interactive quizzes. Websites like Khan Academy, Coursera, and edX offer high-quality educational content on atomic theory, often led by experts in the field. These platforms also provide opportunities for hands-on learning through practical assignments and assessments.
Interactive Simulations and Tools
For those who prefer a more visual and interactive approach, there are numerous simulations and virtual labs available. These resources allow learners to experiment with different atomic models and see how particles interact in real-time. Websites like PhET Interactive Simulations offer engaging tools that demonstrate the behavior of atoms and molecules under various conditions. These simulations help bridge the gap between theoretical knowledge and real-world application.
Academic Journals and Research Papers
For advanced learners or those interested in the latest research, academic journals and research papers provide cutting-edge insights into the field. Reading recent studies can offer a deeper understanding of how atomic theory is applied in modern science and technology. Databases like Google Scholar and PubMed are excellent resources for accessing peer-reviewed papers and research articles.
By utilizing these resources, students and enthusiasts can develop a robust understanding of matter and its interactions, preparing them for further study or practical application in various scientific fields.