
The study of how substances, particularly water, move through different environments plays a crucial role in understanding cellular processes. When certain materials are placed in various solutions, their behavior can tell us much about their properties and the mechanisms at work. This concept is fundamental in many fields of science and helps explain essential biological functions.
Exploring how cells interact with their surroundings provides valuable insights into the processes that sustain life. By examining how water moves in and out of cells, students gain a deeper understanding of how organisms maintain balance and regulate internal conditions. These principles are not only essential for students but also for anyone interested in the mechanics of living systems.
In this section, we will explore the key concepts and procedures involved in studying this type of movement. Through careful observation and analysis, we can better understand the factors that influence these natural processes and their implications for broader biological systems.
Understanding Osmosis in AP Biology
The movement of water through different environments is a vital concept that helps explain various processes within living organisms. This phenomenon occurs when water moves from areas of low solute concentration to areas of high solute concentration, aiming to achieve balance. Understanding this process is fundamental for students as it directly impacts cellular functions and overall homeostasis in living systems.
In experiments, students observe how materials respond when placed in solutions with different concentrations. This helps illustrate how cells interact with their surroundings and maintain internal stability. By mastering the principles of water movement, learners can apply this knowledge to broader biological systems and understand the mechanisms that govern life.
Below is a basic table illustrating the different types of solutions used to study this process and how they affect cells:
| Solution Type | Effect on Cells |
|---|---|
| Isotonic | No net movement of water, cell remains stable |
| Hypotonic | Water enters the cell, causing it to swell |
| Hypertonic | Water exits the cell, causing it to shrink |
Key Concepts of Osmosis for Students

Understanding how substances move across barriers is essential in grasping the core principles of cellular behavior. When studying the movement of water, it is important to know how cells react to changes in their surrounding environments. These reactions are driven by differences in concentration, leading to the movement of molecules in an effort to reach equilibrium.
Students need to focus on the critical factors influencing this movement, including concentration gradients, membrane permeability, and the types of solutions that affect cells. Grasping these key points allows for a deeper understanding of how cells manage their internal conditions and interact with the external environment.
The table below outlines the primary concepts students should be aware of when studying this process:
| Concept | Explanation |
|---|---|
| Concentration Gradient | The difference in concentration of a substance between two areas |
| Selective Permeability | The ability of a membrane to allow certain molecules to pass through |
| Equilibrium | The state in which the concentration of substances is the same on both sides of the membrane |
| Water Potential | The potential energy of water molecules, driving their movement |
Types of Solutions in Osmosis Experiments
When studying the movement of water across membranes, it is crucial to understand how different types of surrounding solutions can affect the behavior of cells. These solutions vary based on the concentration of dissolved substances, and each type can have a distinct impact on the internal structure of a cell. By using these different solutions, students can observe how cells respond under various conditions and gain insights into the mechanics of water movement.
The primary types of solutions used in experiments are categorized based on the concentration of solutes in relation to the cell’s internal environment. Understanding these categories helps in predicting how cells will react, whether they will gain or lose water, or maintain their shape. Below are the key types of solutions and their effects on cells:
| Solution Type | Effect on Cells |
|---|---|
| Isotonic | Water moves in and out at equal rates, cell size remains constant |
| Hypotonic | Water enters the cell, causing it to swell and potentially burst |
| Hypertonic | Water leaves the cell, causing it to shrink and become dehydrated |
How Osmosis Affects Cell Structure

The movement of water into and out of cells plays a crucial role in maintaining their structure and function. As water moves across cellular membranes, it can lead to changes in the shape and size of the cell, depending on the surrounding environment. These changes are vital for the cell’s ability to maintain homeostasis and perform its necessary functions.
In particular, when cells are exposed to different types of solutions, they can either swell, shrink, or remain stable. The balance of water movement helps regulate internal pressure, which is essential for the cell’s structural integrity. Understanding how cells react in various environments can provide valuable insights into the mechanics of cellular processes.
Step-by-Step Osmosis Lab Procedure
Understanding how water moves through membranes involves a clear and systematic experimental process. Following a structured method ensures accurate observations and meaningful conclusions. By carefully setting up and executing each step, students can explore the principles of water movement and its effects on cells.
- Gather materials: Prepare solutions of varying concentrations, a semi-permeable membrane or suitable alternative, containers, and tools for measuring volume and mass.
- Prepare solutions: Mix solutions with different amounts of dissolved substances to create environments for testing. Label each container for clarity.
- Set up the experiment: Place the membrane or sample into each solution. Ensure the samples are fully immersed for uniform exposure.
- Measure initial properties: Record the starting mass or volume of the samples to track changes accurately.
- Observe changes: Allow sufficient time for the process to occur. Note any visible effects, such as swelling or shrinking.
- Record final measurements: Weigh or measure the samples again and compare with the initial values to quantify changes.
- Analyze results: Evaluate the data to understand how the samples responded to different solutions. Relate findings to the underlying principles of water movement.
By following this procedure, students can gain hands-on experience and a deeper understanding of how environmental conditions affect cellular processes. Proper documentation and observation are key to drawing accurate conclusions.
Predicting Results in Osmosis Experiments

Before conducting experiments on water movement, it is important to anticipate how cells will behave in different solutions. Predicting the outcome based on the concentration of solutes in the environment allows students to form hypotheses about how water will move and affect the cells. By understanding the principles behind these predictions, students can better interpret their results and draw meaningful conclusions.
In most cases, the expected outcomes depend on the type of solution the cells are exposed to. If the concentration of dissolved substances outside the cell is higher than inside, water will typically move out, causing the cell to shrink. Conversely, if the external concentration is lower, water will move in, causing the cell to swell. Knowing these patterns helps students to predict the behavior of cells with greater accuracy.
Below is a table showing typical predictions based on different solution types and their effects on cells:
| Solution Type | Predicted Outcome |
|---|---|
| Isotonic | No significant change in cell size, water moves in and out equally |
| Hypotonic | Cell swells as water enters, potential for bursting |
| Hypertonic | Cell shrinks as water exits |
By predicting the results beforehand, students can confirm their hypotheses and understand the role of concentration gradients in cellular function.
Common Mistakes in Osmosis Labs
During experiments involving water movement across membranes, there are several common errors that students often make, which can lead to inaccurate results and confusion. These mistakes may stem from improper setup, incorrect measurements, or a lack of attention to detail. Understanding these pitfalls is essential to conducting a successful experiment and interpreting the findings correctly.
Incorrect Solution Concentrations
One of the most frequent mistakes is not preparing solutions with the correct concentration of dissolved substances. If the concentration is too high or too low, it can affect the results, leading to incorrect conclusions about how water moves in and out of cells. Ensuring the correct ratios and measurements is critical for accurate observations.
Improper Timing and Measurements
Another common mistake is not giving the samples enough time to interact with the solutions. Without adequate time for the water to move, the changes in cell size may not be noticeable. Additionally, failing to measure the initial and final properties of the samples accurately can lead to erroneous data, making it difficult to draw valid conclusions.
Below is a table summarizing some of the most common mistakes and their potential consequences:
| Mistake | Consequence |
|---|---|
| Incorrect solution concentration | Leads to unreliable results and inaccurate understanding of water movement |
| Not allowing enough time for the experiment | Results may show little to no change, leading to incomplete data |
| Failing to measure samples correctly | Results may not reflect the true changes in cell size or mass |
By avoiding these common mistakes, students can improve the accuracy of their experiments and gain a clearer understanding of the processes they are studying.
Analyzing Osmosis Lab Data
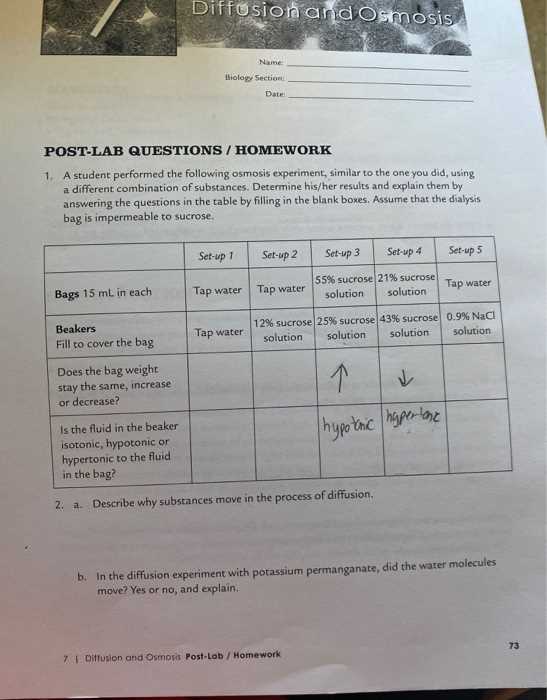
Once the experiment is complete, analyzing the collected data is a critical step in understanding the behavior of water and the effects on cells. By examining changes in the samples, students can draw conclusions about the process of water movement and how it is influenced by the surrounding environment. Proper analysis helps validate hypotheses and provides insights into the underlying principles at play.
To effectively analyze the data, consider the following steps:
- Review the initial and final measurements: Compare the mass, volume, or size of the samples before and after exposure to the solutions. This will show the extent of change.
- Identify trends: Look for patterns in the data, such as whether cells increased or decreased in size depending on the type of solution used.
- Consider external factors: Take into account any environmental factors that may have influenced the results, such as temperature or timing.
- Make comparisons: Compare the outcomes of different solutions to understand the relationship between concentration and cell response.
For example, if the samples placed in a solution with a higher concentration of dissolved substances show a decrease in size, while those in a lower concentration solution increase in size, it supports the idea that water moves from areas of low solute concentration to high solute concentration. This behavior is a key observation when interpreting the results of experiments.
Analyzing data in this systematic way helps ensure that students can accurately interpret their findings and gain a deeper understanding of the mechanisms that govern water movement in living organisms.
Understanding Osmotic Pressure in Cells
Osmotic pressure plays a vital role in the movement of water across cell membranes, influencing how cells maintain their shape and function. This pressure is generated when water moves into or out of the cell due to differences in solute concentration between the inside of the cell and the surrounding environment. Understanding osmotic pressure is key to explaining how cells regulate their internal environment and maintain stability.
Osmotic pressure is essentially the force required to prevent water from moving into a solution. In cells, this pressure is important because it helps maintain cell volume and pressure within the cell walls. If the osmotic pressure is too high or too low, it can disrupt cellular processes and cause cells to become too rigid or too weak.
To understand how osmotic pressure affects cells, consider the following factors:
- Concentration gradient: The difference in solute concentration inside the cell compared to the external environment is the primary factor that drives water movement.
- Cell membrane permeability: The ability of the cell membrane to allow water to pass through determines how easily water can flow into or out of the cell.
- External solution type: Whether the surrounding solution is isotonic, hypotonic, or hypertonic will determine how the osmotic pressure influences the cell’s size and shape.
For example, in a hypotonic solution, where the concentration of solutes is lower outside the cell, water enters the cell, increasing internal pressure. In contrast, in a hypertonic solution, where the solute concentration is higher outside the cell, water exits the cell, causing it to shrink. These movements are all driven by osmotic pressure and have important consequences for the cell’s function.
By understanding osmotic pressure, students can better comprehend how cells interact with their environment and how they maintain their internal balance through selective water movement.
Factors Influencing Osmosis Rates
The rate at which water moves through membranes is influenced by a variety of factors, each contributing to the efficiency and direction of water flow. Understanding these factors is essential for interpreting how cells interact with their environment and manage water balance. These factors can significantly alter the results of experiments, making it important to control or account for them during analysis.
Concentration Gradient
The concentration difference between two solutions on either side of a membrane is a major factor that dictates the movement of water. A larger gradient, where one solution has much more solute than the other, leads to a faster rate of water movement. This is because water moves toward the higher solute concentration in an attempt to balance the concentrations across the membrane.
Temperature
Temperature plays a crucial role in the rate of water movement. As temperature increases, the molecules in the solution move faster, which can speed up the process of water passing through the membrane. Higher temperatures generally increase the rate of diffusion, while lower temperatures slow it down, resulting in less movement.
Other factors that influence water movement include:
- Membrane Permeability: The easier it is for water molecules to pass through the membrane, the faster the movement will occur.
- Surface Area: A larger surface area allows more water to pass through at once, accelerating the rate of movement.
- Pressure: Increasing pressure on the solution can also speed up the movement of water, as it forces molecules through the membrane more quickly.
By considering these factors, students can predict and control the movement of water more accurately during experiments, leading to more reliable results and deeper insights into the behavior of cells.
Osmosis and Its Role in Homeostasis
Water movement across cell membranes is crucial for maintaining the balance of internal conditions within an organism. This process helps regulate the distribution of nutrients and the removal of waste products, playing a significant role in keeping the environment inside cells stable. Without proper regulation of water flow, cells could become damaged or lose their ability to function effectively.
The movement of water is primarily driven by differences in solute concentrations, which ensures that cells maintain their volume and pressure within healthy limits. By controlling the flow of water in and out of cells, organisms are able to keep internal conditions, such as temperature and ion concentration, at optimal levels for survival.
Key ways in which water movement supports homeostasis include:
- Regulation of cell volume: Water entering or leaving the cell helps maintain a consistent cell size, preventing it from swelling or collapsing.
- Ion balance: The movement of water also helps to distribute ions and maintain their concentrations within the cell, which is essential for proper cellular function.
- Removal of waste: Water transport aids in the elimination of metabolic byproducts, helping the cell maintain its internal health.
In summary, the movement of water plays an essential role in ensuring that cells and organisms function efficiently, supporting homeostasis by maintaining a stable internal environment despite external fluctuations.
Effect of Temperature on Osmosis
Temperature plays a significant role in the rate at which water moves through membranes. As the temperature changes, it directly affects the kinetic energy of molecules, which in turn influences the movement of water. Understanding how temperature impacts this process is crucial for interpreting experimental results and understanding biological processes in various environments.
How Temperature Affects Molecule Movement
When the temperature increases, the movement of water molecules becomes faster. This is because molecules gain more kinetic energy, allowing them to move more rapidly across the membrane. As a result, the rate of movement increases, leading to faster equilibrium between solutions on either side of the membrane.
Impact on Membrane Permeability
Higher temperatures can also affect the structure of the cell membrane. At elevated temperatures, the lipid bilayer of the membrane can become more fluid, potentially allowing more molecules, including water, to pass through. However, if the temperature becomes too high, it can damage the membrane, impairing its ability to regulate the flow of water effectively.
Key points to consider about temperature and water movement:
- Increased temperature: Leads to faster movement of molecules, increasing the rate of water transport.
- High temperature: Can cause damage to cell membranes, potentially disrupting cellular processes.
- Low temperature: Slows down molecular movement, leading to a decrease in the rate of water movement across membranes.
In conclusion, temperature is a crucial factor that affects the efficiency of water movement through membranes. Understanding how temperature influences this process helps explain cellular behavior under different environmental conditions.
Water Potential and Its Application
Water potential is a concept that helps explain the movement of water in plants and other organisms. It is a measure of the potential energy of water, which influences how water flows through different environments, such as soil, plant tissues, and cell membranes. By understanding water potential, we can predict how water will move in response to changes in pressure or solute concentration.
This concept is especially important in understanding how water moves from one area to another in plants. For instance, it helps explain how plants take up water from the soil and transport it to different parts of the plant, such as the leaves and roots. The movement of water is governed by gradients in water potential, with water flowing from areas of high potential to areas of lower potential.
Key Factors Affecting Water Potential
Water potential is influenced by several key factors, which together determine how water moves in a given environment:
- Solute Potential: This refers to the effect of dissolved substances in water. The more solutes present, the lower the water potential, as water moves toward areas with higher solute concentration.
- Pressure Potential: This is the physical pressure exerted on water. In plants, pressure potential is often created by turgor pressure in cells, which helps maintain cell rigidity.
- Gravity: In tall plants, gravity can also affect water movement, pulling water downwards and influencing water potential.
Applications of Water Potential
Understanding water potential has practical applications in a variety of biological processes:
- Plant Water Uptake: By understanding the water potential in soil and plant roots, we can predict how plants will absorb water and nutrients from the soil.
- Water Transport in Plants: Water potential helps explain the movement of water from the roots to the leaves, aiding in the process of transpiration and nutrient transport.
- Environmental Impact: Changes in water potential due to environmental factors, such as drought or soil salinity, can affect plant growth and water availability.
In conclusion, water potential plays a critical role in the movement of water within biological systems. By understanding the factors that influence water potential, we can gain deeper insights into how water flows and how organisms maintain internal balance.
Real-Life Examples of Osmosis

In nature, the movement of water across membranes plays a crucial role in maintaining the balance within living organisms. This process is involved in various physiological functions, and we can observe its effects in many real-world situations. Understanding these examples helps to see how this concept is not just a theoretical idea, but a vital part of everyday life in organisms, including humans, plants, and animals.
Water Movement in Plant Cells
One of the most common real-life examples of water movement through membranes occurs in plants. Plants rely on the movement of water from the soil into their roots and through their vascular tissues to maintain hydration and nutrient transport. When a plant’s cells are exposed to environments with differing water concentrations, the movement of water helps to ensure the cells maintain their structure and function. In hypertonic conditions, water moves out of the cells, causing them to shrink, while in hypotonic conditions, water flows into the cells, helping them maintain turgidity and structural integrity.
Human Kidney Function
The kidneys are a great example of how water moves in and out of cells in humans. Through a complex filtration system, the kidneys regulate the body’s fluid and electrolyte balance. In the nephrons of the kidneys, water moves across cell membranes, reabsorbing it into the bloodstream when needed or excreting it in the form of urine. This process helps to maintain the body’s internal environment and ensure that cells receive the right amount of water for proper function.
These examples demonstrate the importance of water movement in both plants and animals, showcasing how this process is integral to maintaining life and ensuring the proper functioning of cells and tissues.
Importance of Accurate Measurements in Labs
In scientific experiments, precision in measurements is critical to ensuring reliable and reproducible results. Whether it’s the amount of a substance, temperature, time, or other variables, small inaccuracies can lead to significant errors in the outcomes of an experiment. Accurate data is the foundation of sound conclusions, and without it, any interpretation of results may be flawed or misleading.
Ensuring Consistency and Reliability
When conducting experiments, the ability to consistently measure variables allows for comparisons between different trials and helps to identify patterns. Precision ensures that results are not just random but reflect the true relationship between the tested factors. Without this consistency, it becomes difficult to determine whether changes in the outcome are due to the experiment itself or simply the result of measurement errors.
Minimizing Experimental Errors
Even small deviations in measurement can lead to large errors, especially when dealing with sensitive biological or chemical reactions. For example, inaccurate measurement of solutions in an experiment can alter the concentrations, affecting how substances interact with each other. This can lead to incorrect conclusions, wasted time, and resources. Therefore, using calibrated equipment, such as precise balances, thermometers, and volumetric tools, is essential to minimize such errors and ensure valid results.
Ultimately, accurate measurements not only uphold the integrity of the experiment but also contribute to the advancement of scientific knowledge. Whether working in research, education, or industry, precision is a cornerstone of good scientific practice.
Experimental Setup for Osmosis Labs
Setting up an experiment to observe the movement of substances through membranes requires careful planning and organization of materials. The experimental design plays a vital role in ensuring that the results are accurate and meaningful. A well-structured setup helps researchers test specific hypotheses while controlling variables that might otherwise introduce errors into the findings. The procedure typically involves selecting the appropriate materials and preparing them in a manner that allows for consistent observation and measurement.
Materials and Equipment
For a successful experiment, a variety of materials are needed, depending on the specific goals of the study. Commonly used equipment includes:
- Transparent containers (beakers, test tubes, etc.)
- Membrane materials (such as dialysis tubing or selectively permeable membranes)
- Measuring tools (scales, pipettes, rulers for measurement accuracy)
- Solutions of varying concentrations (for testing different conditions)
- Timer or stopwatch (to track the duration of the process)
Preparing the Setup

The preparation process is essential for ensuring consistency across trials. Begin by filling the containers with the selected solutions. Carefully seal the membranes around the test substances, ensuring no leaks occur that could affect the integrity of the experiment. Depending on the hypothesis, place the containers in a controlled environment where factors such as temperature can be regulated. It is crucial to document the initial conditions of the experiment (e.g., solution concentration, container size, and duration) to allow for accurate analysis and reproducibility of results.
Once the setup is in place, the experiment can proceed, and the changes in the systems can be observed and measured over time. By maintaining control over the variables, researchers can gain a clearer understanding of the phenomena under investigation.