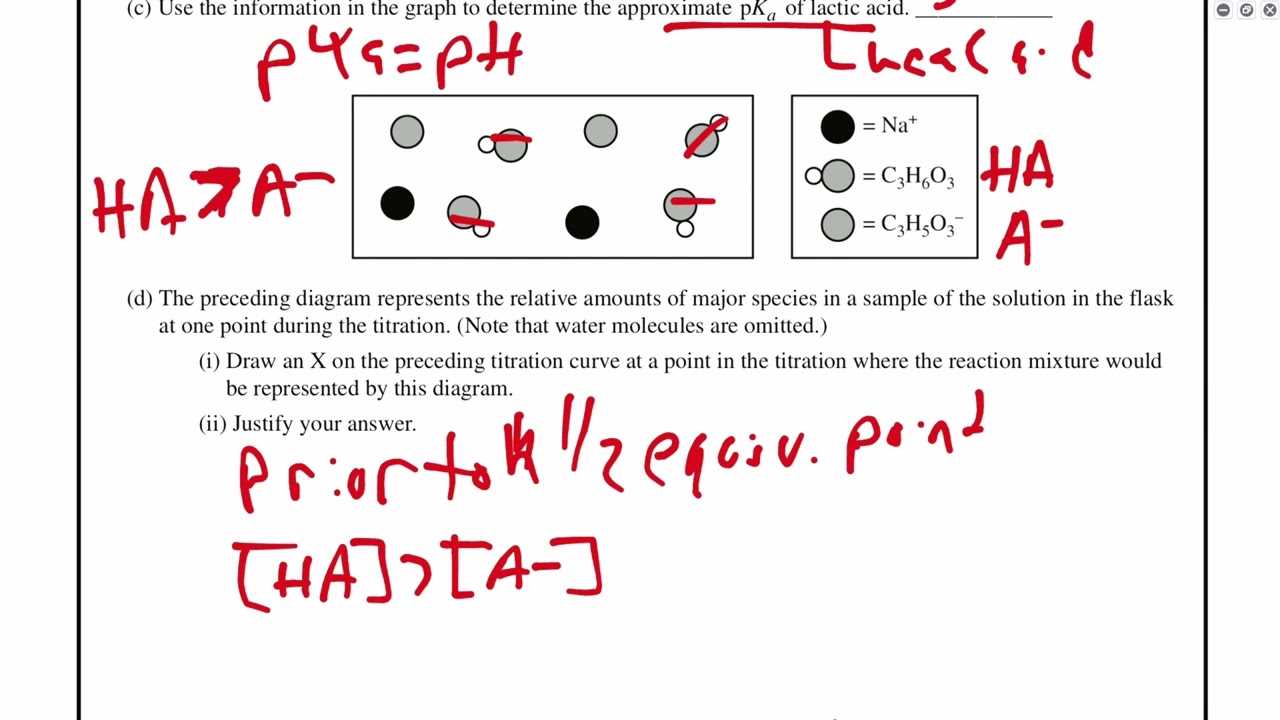
In this section, we will explore the essential concepts and problem-solving techniques required for excelling in the AP exam’s electrochemical topics. A strong grasp of these principles is crucial for tackling complex questions that test your ability to understand and apply various scientific phenomena.
The exam challenges students to demonstrate their understanding of how energy is transferred in chemical processes, focusing on reactions that involve electron flow and energy changes. With the right approach, you can approach these questions with confidence, accurately solving problems and articulating your reasoning effectively.
By breaking down key elements, such as how to manage redox reactions or interpret cell potentials, we aim to provide clear strategies and detailed explanations that will help you perform well under exam conditions. Familiarizing yourself with these techniques will ensure you’re fully prepared to tackle even the most challenging parts of the test.
AP Chemistry Electrochemistry Free Response Answers
In this section, we will focus on effective strategies for tackling complex questions related to energy transfer and electron flow. These topics often appear in various formats during the exam and require a deep understanding of underlying principles. The goal is to help you approach these tasks with clarity and confidence, ensuring you can accurately explain your reasoning and solve problems systematically.
Key to success is understanding how to navigate the intricate relationships between different components, such as half-reactions, cell potentials, and the role of concentrations in driving reactions. Mastering these concepts will allow you to break down even the most challenging problems and offer well-supported conclusions, demonstrating your expertise in the subject.
Furthermore, knowing how to structure your responses effectively is crucial. Clear, concise explanations that connect theoretical knowledge with practical problem-solving will make a strong impression. Whether dealing with standard potentials or analyzing redox processes, a structured approach ensures that you can communicate your thought process clearly while addressing all parts of the question.
Understanding Electrochemistry in AP Chemistry
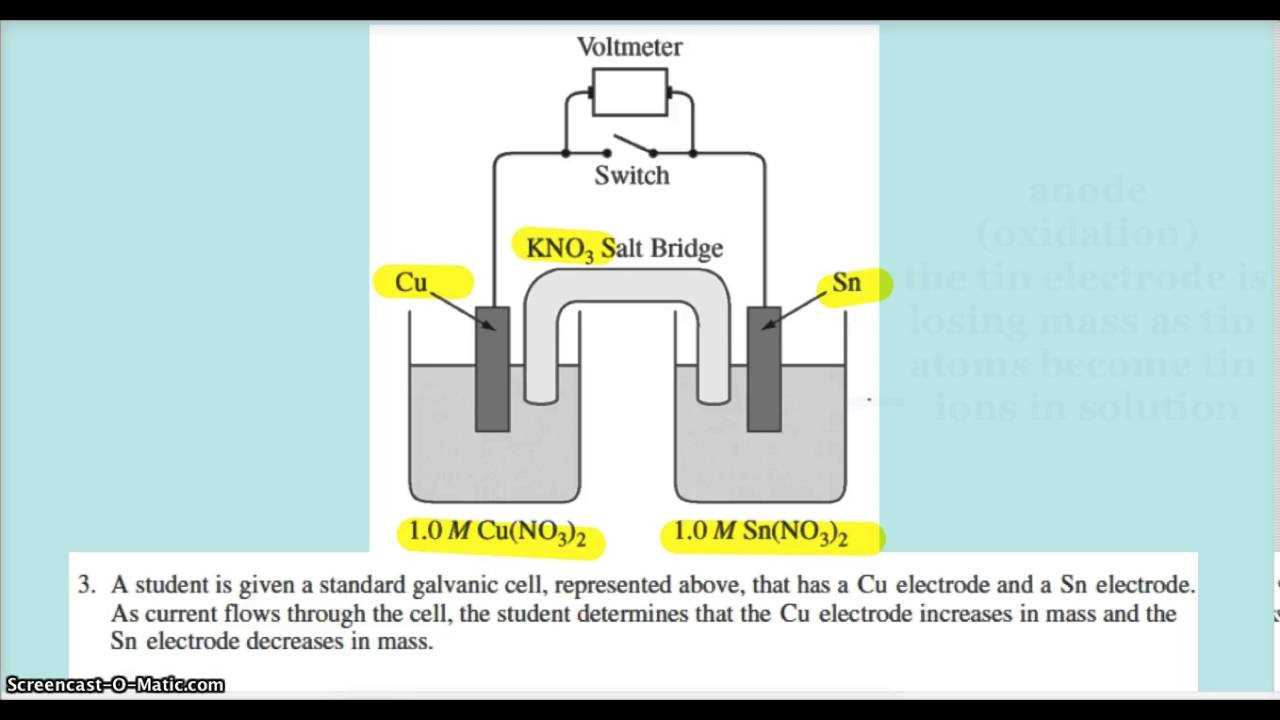
Grasping the core concepts behind energy transfer and electron movement is essential for succeeding in the AP exam. This area of study delves into how certain reactions produce electrical energy or how electricity can be used to drive chemical changes. Understanding these processes not only helps with exam questions but also with appreciating the real-world applications, such as in batteries and electroplating.
The Role of Redox Reactions
Redox reactions are at the heart of this topic. These reactions involve the transfer of electrons between substances, with one substance being oxidized and the other reduced. Knowing how to identify these changes is fundamental to solving problems in this area.
Electrochemical Cells and Their Functions
Electrochemical cells are devices that convert chemical energy into electrical energy, or vice versa. There are two main types: galvanic cells, which produce energy, and electrolytic cells, which require an external source of power. Understanding the structure and operation of these cells is crucial for tackling questions on the subject.
| Cell Type | Purpose | Example |
|---|---|---|
| Galvanic Cell | Produces electrical energy from spontaneous reactions | Battery |
| Electrolytic Cell | Requires external power to drive non-spontaneous reactions | Electroplating |
In the context of the AP exam, it’s important to practice identifying and analyzing both types of cells, as questions often require comparing and contrasting their properties and functions. By mastering these concepts, students can confidently solve a wide range of problems related to energy conversion and electron flow.
Key Concepts in Electrochemistry for AP Exam

Mastering the core principles related to electron transfer and energy conversion is essential for tackling questions on this topic in the AP exam. The ability to identify key concepts and apply them accurately will ensure a comprehensive understanding of the subject. This section highlights the fundamental ideas that form the basis for answering related questions effectively.
Important Principles to Remember
- Redox Reactions: These reactions involve the transfer of electrons, with one substance being oxidized and another reduced.
- Cell Potential: The ability of a cell to drive an electrical current, which depends on the difference in potential energy between two half-reactions.
- Half-Reactions: Understanding how to break down reactions into oxidation and reduction half-equations is crucial for solving problems.
- Standard Electrode Potentials: These values help predict the direction of electron flow in a given system.
Types of Electrochemical Cells
- Galvanic Cells: Devices that generate electrical energy from spontaneous reactions.
- Electrolytic Cells: Use external energy sources to drive non-spontaneous reactions.
- Concentration Cells: Electrochemical cells where the electrolyte concentrations differ between the two half-cells.
These fundamental concepts are often tested in a variety of formats during the exam, so it’s important to become comfortable with each idea and how they interact. By reviewing and practicing with these principles, you will be prepared to approach questions with confidence and precision.
Common Mistakes in Free Response Questions
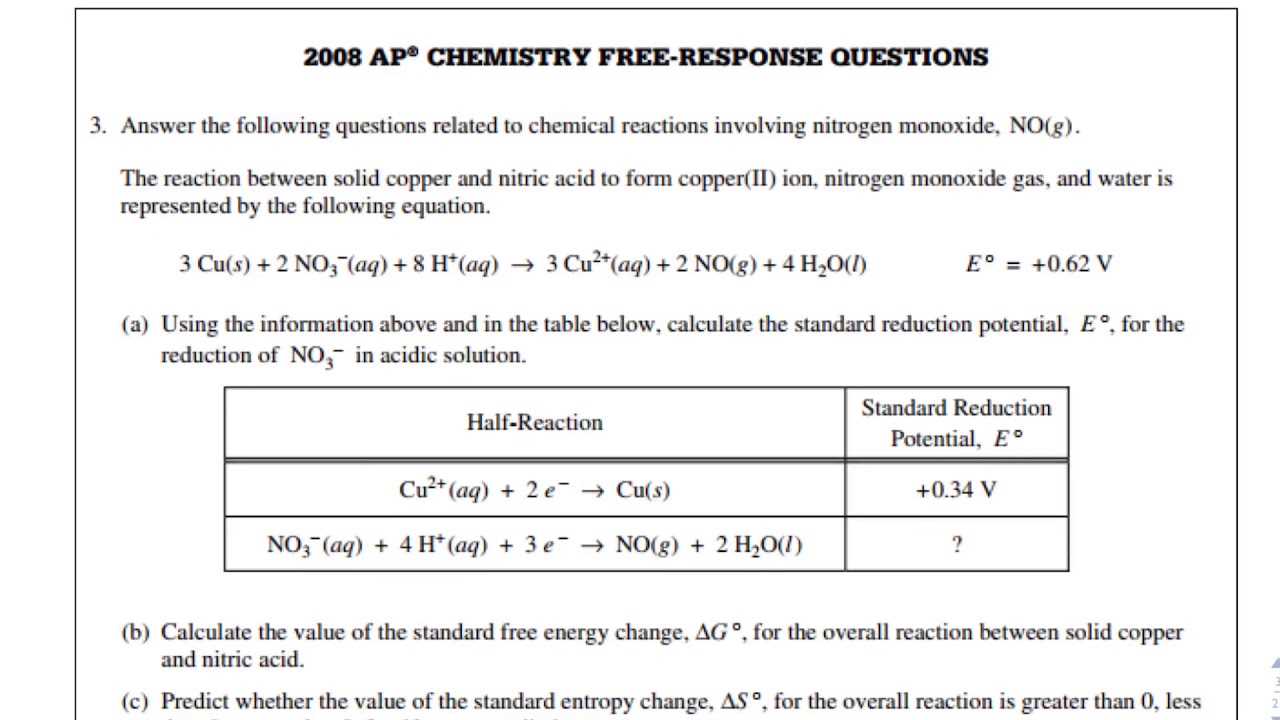
When tackling complex questions in the AP exam, students often make small errors that can significantly affect their overall score. These mistakes typically arise from misinterpretations of the problem, improper application of concepts, or missing critical details. By understanding and avoiding these common pitfalls, you can improve both your accuracy and efficiency in answering questions related to energy transfer and electron movement.
Misunderstanding the Question Requirements
One of the most frequent mistakes is failing to fully understand what the question is asking. Sometimes, students focus on one aspect of the problem and overlook others. This can lead to incomplete or incorrect responses.
- Skipping units: Failing to include correct units in calculations or final answers can result in lost points.
- Missing key terms: Often, specific terms like “oxidation” or “reduction” must be explicitly mentioned, but students sometimes overlook this detail.
- Not answering all parts: Many questions have multiple components, and missing one part can lead to significant deductions.
Incorrect Application of Concepts
Another common mistake is using formulas or concepts incorrectly. Even if you understand the basic principles, misapplying them can lead to wrong calculations or conclusions.
- Incorrect balancing: Balancing redox reactions is tricky, and errors in electron balancing can lead to inaccurate results.
- Misinterpreting cell potentials: Confusing the direction of electron flow or the standard electrode potentials can skew your final results.
- Failure to use the Nernst equation: For questions involving concentrations and temperature, neglecting to apply the Nernst equation can result in incorrect values for cell potential.
By carefully reading the question, applying concepts correctly, and checking your work for small errors, you can avoid these common mistakes and ensure a more accurate and complete response.
Strategies for Answering Electrochemistry Questions
Approaching questions related to energy transformation and electron flow requires careful planning and strategic thinking. To perform well in the AP exam, it’s crucial to break down each question into manageable steps, apply the correct principles, and double-check all calculations. Developing a systematic approach will not only help you avoid common mistakes but also ensure that you clearly communicate your reasoning.
Step-by-Step Approach
Start by reading the question carefully to understand what is being asked. Identify key components such as the substances involved, the type of reaction, and any given data. Once the problem is clear, break it into smaller tasks that can be addressed one at a time.
- Balance the reaction: Begin by balancing the half-reactions for oxidation and reduction.
- Identify electrode potentials: Use the standard electrode potentials to determine the direction of electron flow.
- Calculate cell potential: Apply the appropriate formula to calculate the cell potential, keeping track of all units.
Double-Check Work and Units
After completing the calculation, always recheck your work. Ensure that units are consistent throughout the process and that all steps have been completed correctly. Small errors in unit conversions or sign conventions can lead to incorrect answers, even if the overall concept was understood.
- Check for unit consistency: Ensure that all units match throughout the calculation.
- Re-examine the final answer: Recheck your cell potential or any other final value to confirm it aligns with expectations.
By following these strategies, you can improve your accuracy and ensure that your responses are both clear and comprehensive.
How to Balance Redox Reactions Efficiently
Balancing redox reactions is a fundamental skill when working with reactions that involve the transfer of electrons. The process can seem complex, but with a structured approach, you can simplify it and ensure accuracy. Understanding the essential steps and following a systematic method allows you to efficiently balance these reactions and avoid common mistakes.
The key to success is breaking the reaction into two half-reactions: one for oxidation and one for reduction. Once these half-reactions are balanced individually, they can be combined to form the overall balanced equation. By focusing on the electron flow and ensuring mass and charge balance in each half-reaction, you can achieve a correctly balanced reaction.
Step-by-Step Method
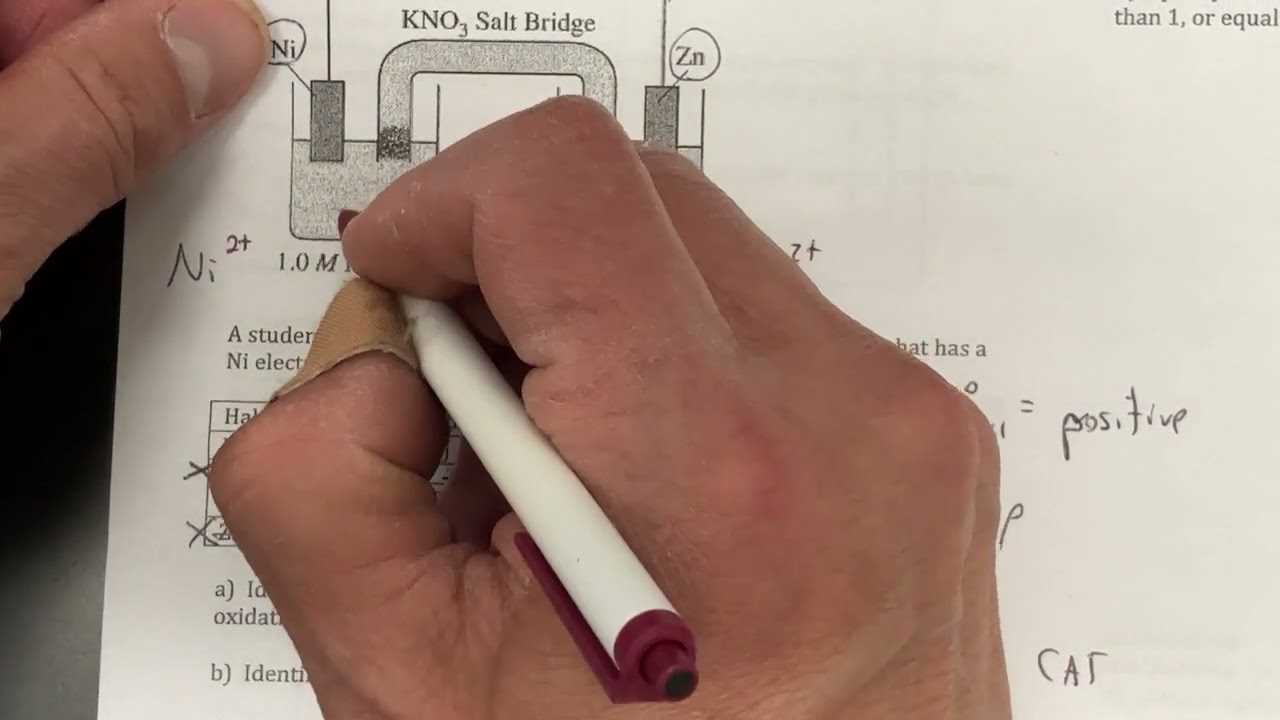
- Write the unbalanced equation: Start with the given reactants and products.
- Separate into half-reactions: Identify what is oxidized and what is reduced.
- Balance atoms other than oxygen and hydrogen: Ensure that all elements are balanced between the two half-reactions.
- Balance oxygen atoms: Add H2O molecules as needed to balance oxygen atoms in both half-reactions.
- Balance hydrogen atoms: Add H+ ions to balance hydrogen atoms in the reaction.
- Balance charge by adding electrons: Ensure that the charge is balanced on both sides of each half-reaction by adding electrons.
- Combine the half-reactions: Multiply each half-reaction by appropriate factors to ensure the number of electrons is the same, then combine them.
- Check balance: Confirm that both mass and charge are balanced in the final equation.
By following this method, you can quickly and accurately balance redox reactions. Practice with various types of reactions will further enhance your ability to tackle these problems with confidence during the AP exam.
Electrochemical Cells and Their Components
Understanding the structure and function of electrochemical cells is essential for solving problems related to energy conversion in reactions. These devices convert chemical energy into electrical energy through the movement of electrons between two half-cells. Each component plays a crucial role in ensuring that the process runs smoothly, and understanding their individual functions is key to mastering this topic.
Basic Components of Electrochemical Cells

- Electrodes: The conductive materials where oxidation and reduction reactions occur. One electrode is the anode (oxidation), and the other is the cathode (reduction).
- Electrolyte: The ionic solution that facilitates the flow of ions between the half-cells and maintains charge balance.
- Salt Bridge: A pathway that allows the movement of ions between the two half-cells to maintain electrical neutrality.
- External Circuit: The conductor that connects the two electrodes, allowing the flow of electrons from one electrode to the other, creating an electric current.
Types of Electrochemical Cells
- Galvanic Cells: Cells that generate electrical energy through spontaneous reactions.
- Electrolytic Cells: Cells that use an external power source to drive non-spontaneous reactions.
- Concentration Cells: A type of galvanic cell where the two half-cells have different concentrations of the same electrolyte.
Each of these components works in harmony to facilitate electron flow and the conversion of energy. By understanding the role of each part, you can better analyze the behavior of electrochemical cells and apply this knowledge to a variety of problems.
Understanding Standard Reduction Potentials

Standard reduction potentials are essential for predicting the direction of electron flow in various reactions. These values provide insight into the tendency of a substance to gain electrons and be reduced. By comparing the standard potentials of different half-reactions, you can determine which reactions will occur spontaneously under standard conditions.
What are Standard Reduction Potentials?
The standard reduction potential is the voltage associated with the reduction half-reaction of a substance when measured under standard conditions, typically 1 M concentration, 1 atm pressure, and 25°C. A more positive potential indicates a greater tendency to gain electrons, while a more negative value suggests a weaker tendency to be reduced.
- Positive values: Substances with positive potentials are stronger oxidizing agents, meaning they readily gain electrons.
- Negative values: Substances with negative potentials are stronger reducing agents, meaning they lose electrons more easily.
How to Use Standard Potentials in Reactions
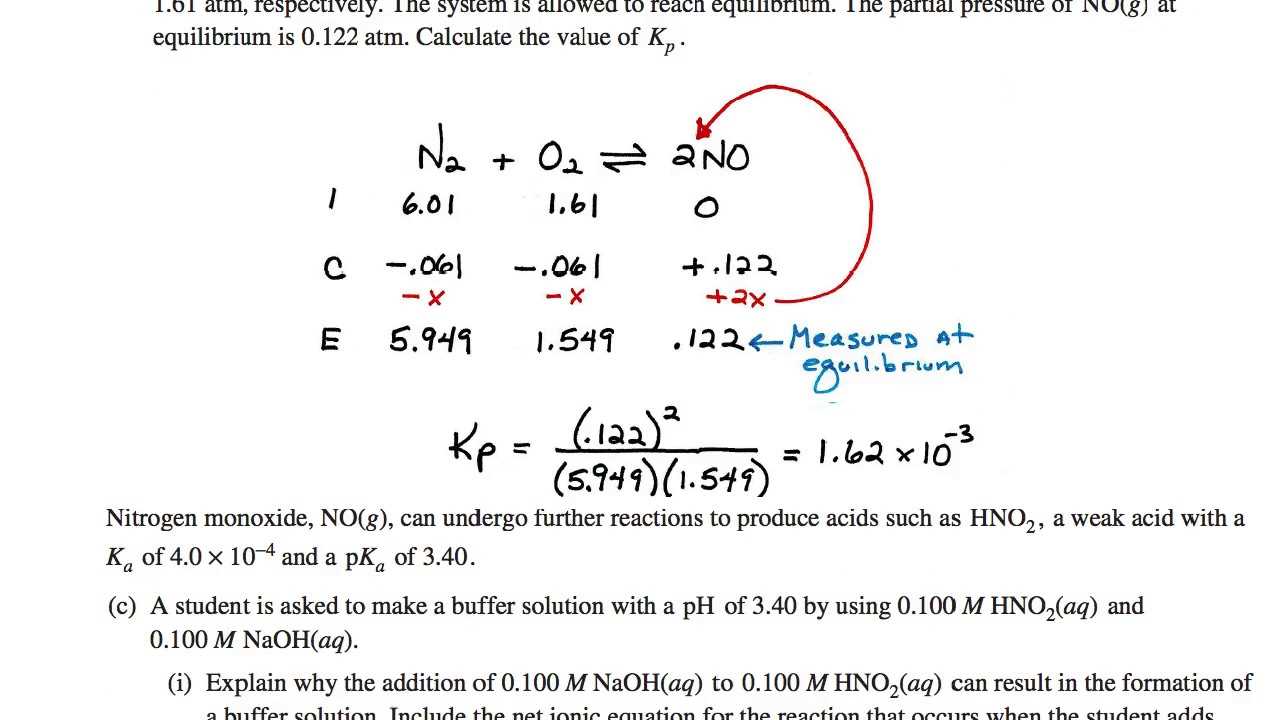
By knowing the standard reduction potentials of the half-reactions involved, you can predict which reaction will proceed spontaneously. To calculate the overall cell potential, subtract the reduction potential of the anode from the reduction potential of the cathode. If the result is positive, the reaction is spontaneous.
- Cell potential equation: Ecell = Ecathode – Eanode
- Spontaneous reactions: Reactions where the cell potential is positive (Ecell > 0).
Mastering the concept of standard reduction potentials is a powerful tool for analyzing and predicting the behavior of reactions in various contexts, from galvanic cells to industrial processes.
Identifying Half-Reactions in Electrochemistry
In reactions involving electron transfer, it is essential to separate the overall reaction into two distinct parts: one where electrons are lost and one where electrons are gained. These two components, called half-reactions, provide insight into the processes occurring at each electrode. Properly identifying and balancing these half-reactions is key to understanding how energy is produced and transferred in a system.
Oxidation and Reduction Half-Reactions
Every electron transfer reaction can be split into two parts: the oxidation half-reaction, where electrons are released, and the reduction half-reaction, where electrons are accepted. Each half-reaction occurs at a different electrode in an electrochemical cell.
- Oxidation: This is the process where a substance loses electrons, typically occurring at the anode.
- Reduction: This is the process where a substance gains electrons, typically occurring at the cathode.
How to Identify Half-Reactions
To identify the half-reactions, begin by examining the full reaction and separating it into the components that involve electron transfer. Focus on the species being oxidized and reduced, and write the half-reactions by balancing atoms and charges. It’s crucial to identify which species lose electrons (oxidized) and which gain electrons (reduced).
- Oxidized Species: Look for the substance that increases its oxidation state, indicating a loss of electrons.
- Reduced Species: Look for the substance that decreases its oxidation state, indicating a gain of electrons.
Once the half-reactions are written, balance the atoms involved (except for hydrogen and oxygen), and then balance charge by adding electrons. This ensures the equation reflects the proper transfer of electrons in the reaction.
How to Calculate Cell Potential
Calculating the potential of an electrochemical cell is crucial for understanding the energy changes that occur during redox reactions. The cell potential, also known as the electromotive force (EMF), is a measure of the driving force behind the flow of electrons in a circuit. This value can be calculated using standard electrode potentials and applying them to the specific reactions taking place at the anode and cathode.
Understanding the Formula
To calculate the overall cell potential, use the following formula:
| Equation | Description |
|---|---|
| Ecell = Ecathode – Eanode | Subtract the standard reduction potential of the anode from that of the cathode to determine the overall cell potential. |
Here, Ecathode represents the reduction potential at the cathode, while Eanode is the reduction potential at the anode. The values for these potentials can be found in standard reduction potential tables.
Step-by-Step Calculation
Follow these steps to calculate the cell potential:
- 1. Identify the half-reactions involved at the anode and cathode.
- 2. Look up the standard reduction potentials for each half-reaction.
- 3. Calculate the difference between the cathode and anode potentials using the formula above.
- 4. If the result is positive, the reaction is spontaneous; if negative, the reaction is non-spontaneous.
For example, if the cathode has a potential of +0.80 V and the anode has a potential of +0.34 V, the cell potential would be:
Ecell = 0.80 V – 0.34 V = 0.46 V
This positive value indicates that the reaction is spontaneous under standard conditions.
Applications of Electrochemistry in Real Life
The principles of electron transfer and the behavior of ions in various environments are not just theoretical concepts; they have significant practical applications in numerous industries and everyday technologies. These processes are integral to the functioning of many devices and systems, influencing everything from energy storage to environmental protection. Understanding how these reactions work is crucial for innovation in a wide range of fields.
Energy Storage and Conversion
One of the most well-known applications is in energy storage devices, such as batteries and fuel cells. These systems rely on redox reactions to generate electrical energy from chemical reactions. Common examples include:
- Batteries: Devices like lithium-ion batteries use reversible redox reactions to store and release energy, powering everything from smartphones to electric cars.
- Fuel Cells: Fuel cells convert chemical energy directly into electricity through the reaction of hydrogen and oxygen, offering an efficient and environmentally friendly energy source.
Corrosion Prevention
The process of oxidation is central to the formation of rust and other forms of corrosion, which can weaken materials over time. Techniques such as galvanization, where a metal is coated with zinc to prevent corrosion, make use of these concepts to protect structures and machinery. Other methods include:
- Sacrificial Anodes: In this method, a more easily oxidized metal is placed in contact with the material to be protected, preventing damage by corroding in its place.
- Protective Coatings: Applying protective coatings to metals can shield them from environmental factors that may lead to oxidation.
Environmental Protection and Waste Treatment

Electrochemical processes also play a vital role in environmental protection and the treatment of wastewater. Techniques such as:
- Electrolytic Water Treatment: Electrolysis is used to purify water by breaking down harmful contaminants, making it suitable for reuse or release into the environment.
- Heavy Metal Removal: Electrochemical methods can remove toxic metals from industrial waste, preventing pollution and making the waste safer for disposal.
These real-world applications show how vital the principles of redox reactions are to a wide range of industries, from renewable energy to environmental conservation. The ability to harness electron transfer processes is key to many technological advances and solutions to global challenges.
Tips for Time Management During the Exam
Effective time management is essential when tackling challenging exams, especially when dealing with complex problem-solving tasks. Managing your time well allows you to address all questions thoroughly without feeling rushed, ensuring that you maximize your performance. With the right strategies in place, you can maintain focus, reduce stress, and improve your chances of success.
Prioritize and Plan
The first step is to assess the exam and quickly identify which sections are worth the most points. By allocating time based on the weight of each part, you can ensure that you devote adequate attention to high-value questions. Follow these steps:
- Skim Through the Entire Exam: Begin by scanning all questions to gauge their difficulty and length. This will help you decide which ones to tackle first.
- Set Time Limits: Assign a specific amount of time for each section, ensuring you stay on track and don’t get stuck on any one question.
Efficient Question Handling
While answering, it’s crucial to stay efficient and avoid spending too much time on any one question, especially if you’re unsure of the answer. If you encounter a tough question, consider these tips:
- Move On When Stuck: If you’re spending too much time on a single problem, move to the next one and come back later with a fresh perspective.
- Keep Track of Time: Regularly check the clock to ensure that you’re pacing yourself according to your plan. If needed, adjust your strategy.
By managing your time effectively, you can ensure that you complete all sections of the exam and give each question the attention it deserves. Practicing these strategies will lead to better time control and improve your overall exam experience.
Electrochemistry Questions in Past Exams
Reviewing past exams is a valuable strategy for understanding the types of problems and concepts that are often tested. By analyzing the questions from previous years, you can gain insights into recurring themes and the level of complexity that might be expected. This preparation helps in identifying the key areas to focus on and how to approach similar questions during the actual exam.
Common Question Formats
In past exams, questions related to electrochemical processes typically appear in various formats. Understanding these formats can help you approach the problems systematically. Some common types include:
- Conceptual Questions: These questions test your understanding of theoretical principles and require clear, concise explanations.
- Calculation-Based Questions: These involve numerical problems where you need to apply formulas and principles to solve for specific values.
- Diagram-Based Questions: Here, you may be asked to sketch or interpret diagrams, often related to cell setups or reactions.
Key Topics Covered

While each exam is different, certain topics tend to be more frequently tested. By reviewing past exams, you can pinpoint which areas are emphasized and prepare accordingly. These topics often include:
- Cell Potential Calculations: Determining the voltage of an electrochemical cell is a common question that often appears in different variations.
- Identifying Half-Reactions: Understanding how to break down reactions into their oxidation and reduction components is another common focus.
- Applications of Galvanic Cells: Questions related to real-life uses of electrochemical cells, such as batteries or corrosion, are frequently tested.
By practicing with past exam questions, you not only become familiar with the format and content but also improve your ability to solve problems under time pressure. This strategy is essential for boosting confidence and performance on the actual test.
Understanding Galvanic and Electrolytic Cells
In the study of electrical energy generation and consumption, understanding the operation of different types of cells is essential. These devices, which convert chemical reactions into electrical energy or vice versa, are fundamental in various applications, from powering everyday devices to industrial processes. The two primary types of cells you will encounter are the galvanic and electrolytic cells. Each operates based on distinct principles and serves different purposes, but both play a critical role in energy conversion.
Galvanic Cells
Galvanic cells, also known as voltaic cells, generate electrical energy through spontaneous chemical reactions. These cells are commonly used in batteries, where a chemical reaction produces a flow of electrons, creating an electric current. Key features of galvanic cells include:
- Spontaneous Reactions: The chemical reactions in these cells occur naturally, releasing energy in the form of electricity.
- Two Electrodes: Galvanic cells consist of two electrodes, typically made of metal, where oxidation occurs at the anode and reduction occurs at the cathode.
- Salt Bridge: A salt bridge or porous disk connects the two halves of the cell to maintain electrical neutrality.
- Energy Output: The primary purpose of a galvanic cell is to convert chemical energy into electrical energy, as seen in everyday batteries.
Electrolytic Cells

In contrast, electrolytic cells use electrical energy to drive non-spontaneous reactions. These cells are often used in processes such as electroplating, electrolysis of water, and the extraction of metals. Some important characteristics of electrolytic cells include:
- Non-Spontaneous Reactions: The reactions in electrolytic cells require an external electrical current to occur, as they do not happen on their own.
- Electrodes: Electrodes in electrolytic cells are also involved in oxidation and reduction, but the direction of electron flow is reversed compared to galvanic cells.
- Energy Consumption: Instead of generating electricity, these cells consume it to drive reactions, such as breaking down compounds or plating metals.
Both types of cells play critical roles in technology and industry, but understanding the differences in their operation is crucial for solving related problems in exams and real-world applications. Knowing when to apply each type of cell and understanding their underlying mechanisms will enhance your ability to answer questions efficiently.
Step-by-Step Guide to Solving Electrochemistry Problems
When faced with a question involving electrical energy transformations, having a clear and systematic approach to solving these problems is crucial. These types of questions often require a thorough understanding of several key concepts and the ability to apply them logically. By following a step-by-step guide, you can break down complex problems into manageable parts, ensuring that you approach them with confidence and clarity. Below is a structured method to solve typical problems related to electrical energy conversion.
Step 1: Identify the Type of Problem
The first step is to recognize the type of problem you are dealing with. Some common problems may involve calculating cell potential, determining the direction of electron flow, or balancing redox reactions. Identifying the specific area will help you select the appropriate formulas and methods. Understanding whether you’re dealing with a galvanic or electrolytic process will also guide your approach.
Step 2: Write the Half-Reactions
Breaking down the overall reaction into half-reactions is essential. In redox reactions, oxidation occurs at the anode and reduction occurs at the cathode. Write these reactions separately and ensure they are correctly balanced for mass and charge. You may need to adjust coefficients to ensure that the number of electrons lost in oxidation equals the number gained in reduction.
- Oxidation: The species that loses electrons will undergo oxidation at the anode.
- Reduction: The species that gains electrons will undergo reduction at the cathode.
Step 3: Use the Nernst Equation or Standard Potentials
Once you have identified the half-reactions, the next step is to use the relevant equations to calculate the cell potential. If standard conditions are provided, you can use standard reduction potentials to determine the overall cell potential. If non-standard conditions are given, the Nernst equation can help adjust the cell potential based on temperature, pressure, and concentration.
- Standard Reduction Potentials: Use these to find the cell potential under standard conditions (1 M concentration, 25°C, 1 atm pressure).
- Nernst Equation: This equation can be used to calculate the cell potential when the concentrations of the reacting species differ from standard conditions.
Step 4: Determine the Direction of Electron Flow
Once you have the cell potential, you can determine the direction of electron flow. In a galvanic cell, electrons flow from the anode (where oxidation occurs) to the cathode (where reduction takes place). The flow of electrons will help you understand the reaction dynamics and answer questions regarding the operation of the cell.
Step 5: Final Check and Units
Before finalizing your solution, always check that your half-reactions are correctly balanced, both in terms of mass and charge. Additionally, ensure that the units are consistent throughout your calculations, especially when using constants like the gas constant or Faraday’s constant. A final review of the steps will confirm that all the necessary components of the problem have been addressed and that the calculations are correct.
By following this step-by-step process, you will be able to effectively tackle complex questions, ensuring clarity and accuracy in your answers. Practice applying this method to various types of problems to build confidence and familiarity with the concepts involved.
The Role of Concentration in Cell Potential
The concentration of ions in a system plays a crucial role in determining the overall potential of a cell. As the concentrations of reactants and products change, so does the electrical energy generated by the cell. The relationship between concentration and cell potential is central to understanding how the system’s behavior can vary depending on the amounts of substances involved. This is particularly important when dealing with non-standard conditions, where the concentration of reactants and products deviates from the idealized standard state.
How Concentration Affects the Cell Potential
The concentration of ions in the electrolyte influences the flow of electrons and the ease with which reactions proceed at the electrodes. In general, a higher concentration of reactants will favor the forward reaction, increasing the potential, while a higher concentration of products will reduce the potential. This effect can be quantified using the Nernst equation, which adjusts the cell potential based on the concentrations of the ions involved in the reaction.
- Increased Concentration of Reactants: When reactant concentration increases, the cell potential tends to rise because the system favors the reduction half-reaction, leading to a higher voltage.
- Increased Concentration of Products: Conversely, increasing the concentration of products will drive the reaction backward, decreasing the cell potential.
Understanding the Nernst Equation
The Nernst equation provides a quantitative method for calculating the potential of a cell under non-standard conditions by taking into account the concentration of the species involved. The equation can be used to determine how variations in concentration affect the cell’s electrical output, which is crucial for predicting how a system will behave in real-world conditions.
| Equation | Description |
|---|---|
| E = E° – (RT/nF) ln(Q) | The Nernst equation, where E is the cell potential, E° is the standard electrode potential, R is the gas constant, T is the temperature, n is the number of electrons transferred, F is Faraday’s constant, and Q is the reaction quotient (product concentrations over reactant concentrations). |
By applying the Nernst equation, one can calculate the exact potential of a cell based on real conditions, adjusting for the actual concentrations of the reactants and products. This is particularly useful for understanding how the potential will change during a reaction or as the concentrations shift due to the progress of the reaction.
Interpreting and Analyzing AP Exam Solutions
Effectively interpreting and analyzing solutions in an exam setting is a critical skill that can significantly improve performance. Understanding not just the final result but also the logical steps that lead to that conclusion is essential for mastering complex problems. This section focuses on how to approach and evaluate solutions systematically, ensuring that each step aligns with the core principles of the subject.
Breaking Down the Problem
When examining a solution, it’s important to break down each part of the problem to identify how it relates to the overall concept. Analyzing each step methodically helps in ensuring that the correct methods were applied and that all aspects of the question were addressed. This approach not only helps in understanding the solution but also aids in identifying any mistakes that might have occurred.
- Identify Key Concepts: Recognize the core principles behind the question and how the solution applies these concepts. Are the proper formulas used? Does the solution consider all necessary variables?
- Check for Units and Conversions: Verify that the correct units are used throughout the calculation and that any necessary unit conversions are made correctly. This ensures the final answer is meaningful and accurate.
Evaluating the Logical Flow
One of the most important aspects of interpreting a solution is assessing the logical flow of steps. Each calculation should build on the previous one, leading to a coherent and accurate result. Understanding why each step was taken can reveal insights into whether the reasoning was sound and if the solution method was appropriate.
| Step | Action | Purpose |
|---|---|---|
| Step 1 | Identify key information in the problem. | Establishes the known variables needed to proceed with calculations. |
| Step 2 | Choose the appropriate equations or principles to apply. | Ensures that the correct approach is taken based on the problem’s requirements. |
| Step 3 | Perform calculations and conversions as necessary. | Generates intermediate results that will be used to solve the problem. |
| Step 4 | Check the solution for consistency with the problem’s context. | Ensures the final result is logical and aligns with expectations based on the given conditions. |
By following this structured approach to problem-solving, one can more easily identify errors, refine the method used, and ultimately arrive at a more accurate and precise solution. It also helps in understanding the reasoning behind each step, reinforcing the underlying principles of the subject matter.
How to Review for the AP Test
Effective preparation for the AP exam requires a focused approach, especially when dealing with topics that involve complex concepts and problem-solving skills. Reviewing the core principles, practicing application-based questions, and reinforcing key ideas are essential steps to ensure success. In this section, we will explore how to efficiently review the material related to this subject area, helping you build a solid foundation and gain confidence before the test.
Start by organizing the key topics that are frequently tested. This allows you to focus on the most important areas and prioritize your study time. Break down each topic into manageable sections, ensuring you understand both the theoretical and practical aspects. Reviewing past exam questions and solutions will also provide valuable insight into the types of questions you may encounter and the best strategies for answering them.
Regularly practice solving problems to strengthen your skills. Working through example questions will help you identify common pitfalls and refine your approach to answering questions efficiently. Additionally, paying attention to the underlying principles of each problem will allow you to recognize patterns and connections across different topics, making it easier to apply your knowledge during the exam.