When facing a challenging assessment in the world of science, proper preparation is key to ensuring a strong performance. Whether you’re reviewing key concepts, solving practice problems, or revisiting important principles, the goal is to build a deep understanding of the material and develop effective strategies for tackling various question types.
Focused revision and strategic practice are essential components in mastering the material. The more familiar you become with the core ideas and their applications, the easier it will be to recall them under pressure. This approach helps minimize stress and boosts confidence when it comes time to put your knowledge to the test.
As you prepare, it’s important to approach your studies with a clear plan. Start by identifying the most crucial topics and breaking them down into manageable sections. This will allow you to track your progress and avoid last-minute cramming. With the right mindset and tools, you can perform at your best and achieve the results you’re aiming for.
Chemistry Semester 1 Exam Review
Success in any scientific assessment relies heavily on a comprehensive understanding of foundational topics. To perform well, it’s crucial to master key concepts and the methods used to apply them in various scenarios. By focusing on the essential principles and practicing their use in different contexts, you’ll be well-prepared to tackle any question that comes your way.
During your preparation, prioritize the most critical areas of study. This will help you gain confidence and enhance your ability to recall important information when needed. Rather than attempting to memorize everything, focus on understanding the core ideas and their interconnections. The more you internalize these concepts, the more efficiently you’ll be able to solve problems and answer questions during the assessment.
Essential Concepts to Focus On
To ensure thorough preparation, it’s important to concentrate on the fundamental ideas that form the foundation of the subject. Understanding these key concepts will allow you to navigate more complex problems with ease. By mastering the basics, you can build a strong framework that supports deeper learning and enhances your problem-solving abilities.
Atomic Structure and Bonding
A solid grasp of atomic structure is crucial, as it underpins much of the subject’s content. Pay special attention to the properties of atoms, how they bond, and the different types of interactions that occur between them. Understanding the role of electrons and how elements interact at the atomic level will help you analyze more complex reactions and phenomena.
Periodic Table Trends
The periodic table provides essential insights into the properties and behaviors of elements. Understanding trends such as electronegativity, ionization energy, and atomic radius will give you a deeper understanding of how elements interact in different situations. These trends are vital for predicting chemical reactions and understanding the relationships between different elements.
Key Study Strategies for Success
Achieving top results requires not only understanding the material but also implementing effective study techniques. Focusing on active engagement with the content, creating a structured study plan, and consistently testing your knowledge can significantly enhance your performance. With the right strategies in place, you can approach the material with confidence and improve retention over time.
Effective Time Management
Managing your time efficiently is crucial for success. Break down your study sessions into focused intervals and allocate specific time slots for each topic. Consider using the Pomodoro technique, which involves studying for 25 minutes followed by a 5-minute break, to keep your mind fresh and maintain concentration.
- Create a study schedule with clear goals for each session.
- Prioritize challenging topics and spend more time on them.
- Break down larger topics into smaller, manageable chunks.
Active Learning Techniques

Active learning enhances understanding and helps solidify the material. Engage with the content through various methods like summarizing information in your own words, teaching others, or applying concepts in practice problems. This active approach strengthens neural connections and ensures long-term retention.
- Summarize key ideas and concepts in your own words.
- Use flashcards for quick recall and repetition.
- Work through practice problems to apply what you’ve learned.
Understanding Chemical Reactions Thoroughly
To fully grasp the underlying processes of reactions, it is important to comprehend the changes occurring at both the molecular and atomic levels. By analyzing the interactions between reactants and products, you can better predict the outcomes and understand how different factors influence the reaction. This foundation allows you to approach complex scenarios with clarity and accuracy.
Focus on the different types of reactions and how they proceed. Whether it’s a combustion reaction, a redox process, or an acid-base interaction, each type follows specific patterns that can be recognized and applied. Understanding these patterns provides a structured approach to solving problems and analyzing unknown reactions.
Key Concepts to Explore:
- Reaction rates and what influences the speed of a reaction.
- Activation energy and its role in determining whether a reaction will occur.
- Conservation of mass and how it applies to every reaction, ensuring that atoms are neither created nor destroyed.
Mastering Atomic Structure and Bonding
Understanding the fundamental building blocks of matter is crucial for mastering more advanced topics. The way atoms are structured and how they bond with one another forms the basis of most scientific concepts. By grasping the underlying principles of atomic structure and the forces that drive atoms to connect, you can better predict and analyze the properties and behaviors of different substances.
The structure of an atom consists of a nucleus surrounded by electrons, each with a specific energy level. These electrons play a key role in how atoms interact with one another to form bonds. Understanding the types of bonds, such as covalent, ionic, and metallic, allows you to predict how elements will combine and how those combinations will behave in different environments.
Table of Atomic Subatomic Particles:
| Particle | Charge | Location | Mass |
|---|---|---|---|
| Proton | Positive (+) | Nucleus | 1 amu |
| Neutron | Neutral | Nucleus | 1 amu |
| Electron | Negative (-) | Electron Cloud | Negligible |
By familiarizing yourself with these concepts, you can gain a deeper understanding of how atoms form the substances around us and how these bonds dictate the properties and reactions of matter.
Important Equations to Remember

Mastering key equations is essential for understanding the relationships between different variables in scientific calculations. These formulas provide a structured way to approach problems and offer a reliable method for solving them. By committing the most frequently used equations to memory, you can solve a wide variety of questions efficiently and with confidence.
Many equations serve as the foundation for understanding how energy, matter, and reactions interact. From calculating energy changes during reactions to determining molar relationships, these equations are tools that will help you navigate more complex topics. Make sure to not only memorize them but also understand their applications and derivations.
Key Equations:
| Equation | Description |
|---|---|
| E = mc² | Energy-mass equivalence formula (Albert Einstein) |
| PV = nRT | Ideal gas law, relating pressure, volume, and temperature of a gas |
| C = λν | Relationship between the speed of light (c), wavelength (λ), and frequency (ν) |
| n = m/M | Formula for calculating the number of moles (n) from mass (m) and molar mass (M) |
| ΔH = ΣH(products) – ΣH(reactants) | Enthalpy change formula for chemical reactions |
These formulas not only simplify complex calculations but also reveal important insights into the nature of the substances involved. Understanding their significance is just as important as knowing how to apply them in different scenarios.
Tips for Balancing Chemical Equations
Balancing equations is a crucial skill that ensures the conservation of matter during a reaction. The law of conservation of mass dictates that the number of atoms of each element must be the same on both sides of the equation. Mastering this process is essential for understanding how different substances interact and transform in chemical processes.
To effectively balance an equation, start by writing down the unbalanced formula, making sure to include all reactants and products. Focus on balancing one element at a time, typically starting with elements that appear in the least number of compounds. Once one element is balanced, move to the next. It’s also important to adjust coefficients rather than subscripts to preserve the identity of the compounds involved.
Steps to Follow:
- Write the unbalanced equation with correct chemical formulas.
- Balance atoms that appear in the least number of compounds first.
- Balance oxygen and hydrogen atoms last, as they are often present in multiple compounds.
- Check that the number of atoms of each element is equal on both sides.
- Ensure that all coefficients are in the lowest possible whole numbers.
By practicing these steps and keeping a systematic approach, you will become more efficient at balancing equations and be able to handle more complex reactions with ease.
How to Approach Problem-Solving
Effective problem-solving involves a structured method that allows you to analyze and break down complex questions. By developing a systematic approach, you can tackle even the most difficult problems with confidence. A clear step-by-step process helps in organizing your thoughts and ensures that no crucial details are overlooked.
Step 1: Understand the Problem

The first step in solving any problem is to read the question carefully and ensure that you understand the given information and what is being asked. Break down the problem into manageable parts and identify the known variables and what you need to find.
- Highlight key information in the problem statement.
- Identify any relationships or formulas that might be useful.
- Determine what is being asked and what needs to be solved.
Step 2: Plan Your Approach
Once you understand the problem, the next step is to devise a strategy. This could involve choosing the appropriate formula, setting up an equation, or selecting the method you’ll use to find the solution. Make sure your approach is logical and aligns with the principles you have studied.
- Write down any relevant equations or principles you may need.
- Choose the method that best fits the type of problem (e.g., algebraic manipulation, dimensional analysis).
- Plan the steps you will follow to reach the solution.
By staying organized and methodical, you can approach each problem with a clear sense of direction, making it easier to find the correct solution efficiently.
Reviewing Periodic Table Trends
Understanding the patterns and trends within the periodic table is essential for predicting the properties of elements and their behavior in reactions. The arrangement of elements in the table is not random; it reflects underlying principles that govern atomic structure and behavior. By recognizing these trends, you can gain insights into how different elements interact, their reactivity, and their physical characteristics.
Trends in Atomic Size
One of the most noticeable trends in the periodic table is the change in atomic size as you move across a period or down a group. Atomic radius decreases across a period from left to right due to the increasing positive charge in the nucleus, which pulls electrons closer. Conversely, atomic radius increases as you move down a group because additional electron shells are added, making the atom larger.
- Across a period: Decreases from left to right.
- Down a group: Increases as new electron shells are added.
Trends in Electronegativity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also follows a predictable trend. As you move across a period, electronegativity increases because atoms have more protons and can attract electrons more strongly. On the other hand, as you move down a group, electronegativity decreases due to the increasing distance between the nucleus and the outermost electrons, making it harder to attract electrons.
- Across a period: Increases from left to right.
- Down a group: Decreases as atomic size increases.
By familiarizing yourself with these trends, you can predict how elements will behave in various chemical reactions and understand their relationship to one another in the periodic table.
Common Mistakes to Avoid During the Exam
While taking a test, it’s easy to make small errors that can cost you valuable points. Being aware of the common pitfalls can help you stay focused and avoid mistakes that could negatively impact your performance. A careful approach, along with good preparation, can ensure that you maximize your potential during the assessment.
- Rushing Through Questions: One of the most frequent mistakes is hurrying through the questions without fully understanding them. Take time to read each question carefully, especially if it’s complex or includes multiple parts.
- Not Showing Work: Always show the steps you take to solve a problem, even if you are confident in your answer. Sometimes partial credit is awarded, and showing your reasoning helps the examiner follow your thought process.
- Skipping Difficult Questions: It’s tempting to leave the challenging questions for later, but doing so can lead to time pressure toward the end. Instead, mark difficult questions and return to them after completing easier ones.
Other Common Errors
- Misinterpreting the Units: Pay attention to the units provided in the problem and make sure to convert them when necessary. Using incorrect units can lead to incorrect answers, even if your calculations are right.
- Overlooking Simple Details: Sometimes, answers can be lost by overlooking basic facts like the charge on an atom, the state of a substance, or whether the question asks for a specific value or a range.
- Not Managing Time Wisely: Time management is crucial. Make sure to pace yourself and allocate enough time to answer all questions. Keep an eye on the clock and avoid spending too much time on a single question.
Avoiding these mistakes can significantly improve your performance. Be mindful of your approach, take your time to understand the questions, and double-check your work before submitting your responses.
Effective Time Management for Chemistry Exams
Proper time management is essential for performing well in any assessment. By managing your time wisely, you can ensure that you have enough opportunity to address all questions thoroughly and with confidence. Effective time planning allows you to avoid stress, complete the test without rushing, and maximize your score.
Planning Before the Test
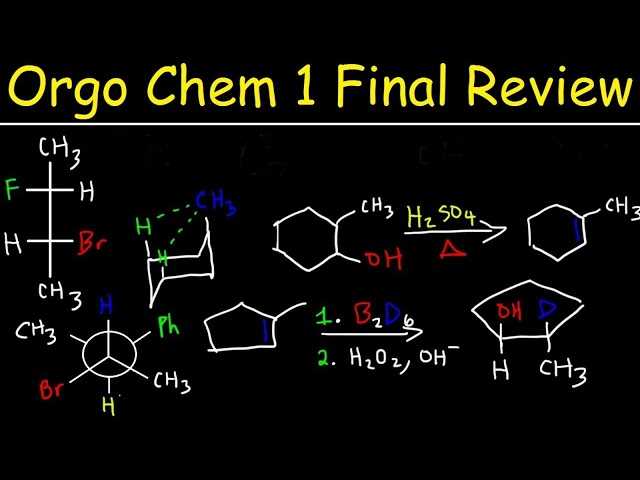
Start by allocating enough time to review all relevant material before the assessment. Prioritize topics based on their difficulty or importance, and make sure you have time for both practice and reviewing key concepts. It’s important to plan well in advance, leaving no last-minute cramming, which can lead to unnecessary stress.
- Set specific goals for each study session (e.g., mastering a topic or completing a set of problems).
- Review weak areas first, then reinforce stronger topics closer to the assessment.
- Take regular breaks to prevent burnout and maintain focus.
Managing Time During the Test
During the assessment, time management is just as important. Start by scanning the entire paper to get an idea of the number of questions and their complexity. Begin with the sections you are most comfortable with, and leave more time-consuming or challenging problems for later. This way, you ensure that you answer all the questions you can with ease before dedicating extra time to more difficult ones.
- Allocate time for each section based on the marks it carries.
- Don’t dwell too long on a single question–move on if you’re stuck and return to it later.
- Always leave a few minutes at the end to review your answers for any mistakes or missed steps.
By following these strategies, you can make the most of the time available to you and approach the assessment with a calm and organized mindset.
Helpful Resources for Last-Minute Studying
When time is running short and the clock is ticking down to your upcoming assessment, having access to the right materials can make all the difference. Leveraging effective resources during the final hours can help reinforce your knowledge, clarify any uncertainties, and ensure you’re prepared for the challenge ahead. Focusing on high-quality study aids is key for efficient last-minute preparation.
Online Tools and Platforms

Online platforms offer a wide variety of resources that are ideal for quick reviews and targeted learning. These tools are accessible and provide the flexibility to study anytime, anywhere. Some of the most effective options include:
- Interactive Quizzes: Websites that offer practice questions based on your subject allow you to test your knowledge under time pressure.
- Video Tutorials: Platforms like YouTube feature concise explanations of complex topics, providing visual and auditory learning.
- Online Flashcards: Flashcard apps are perfect for quick recall of key concepts and terms, helping reinforce memory retention.
Books and Study Guides

If you’re more comfortable with traditional study methods, books and printed guides remain invaluable resources. While in-depth books are great for overall learning, concise study guides are often tailored to help you prepare for specific types of assessments.
- Revision Guides: Focused guides often summarize key ideas and offer practice exercises to test your understanding.
- Past Papers: Reviewing previous assessments can give you insight into the types of questions likely to appear and help you practice effective strategies.
- Textbook Summaries: Many textbooks include summary sections at the end of chapters, which can be useful for a quick recap of important information.
With the right resources at your disposal, even a limited amount of time can be maximized for efficient and focused studying. Prioritize these tools to ensure you feel confident and well-prepared when it’s time to face the challenge.
Understanding Laboratory Techniques and Safety
In any scientific setting, mastering fundamental techniques and ensuring safety protocols are strictly followed is crucial. Effective handling of instruments, chemicals, and procedures can significantly influence the success and accuracy of experiments. Equally important is understanding the risks associated with various tasks and knowing the necessary precautions to keep both individuals and the environment safe. By honing your technical skills and prioritizing safety, you lay the groundwork for reliable results and a secure working environment.
Key Laboratory Techniques
Proficiency in essential methods is vital for carrying out experiments accurately. Here are some of the most important techniques that ensure precision in scientific investigations:
- Accurate Measurement: Whether working with solids, liquids, or gases, using precise instruments and techniques ensures reliable data. Calibration of tools such as balances and pipettes is essential for consistency.
- Proper Mixing and Stirring: Even distribution of substances is critical in ensuring uniform reactions. Know when to stir, shake, or mix to achieve consistent results.
- Heating and Cooling Methods: Temperature control plays a key role in many experiments. It’s crucial to understand how to use heating devices safely and maintain a consistent temperature when necessary.
Laboratory Safety Protocols

Adherence to safety procedures is paramount to avoid accidents and injuries. Whether dealing with hazardous chemicals or sensitive equipment, following the right protocols ensures a safe and productive environment. Here are some essential guidelines:
- Personal Protective Equipment (PPE): Always wear gloves, goggles, lab coats, and other protective gear to shield yourself from potential hazards such as chemical spills or flying debris.
- Familiarity with Emergency Procedures: Knowing the location of emergency equipment (like fire extinguishers and eyewash stations) and the actions to take during an accident is crucial for a quick response in critical situations.
- Safe Handling of Hazardous Materials: Always handle chemicals and other hazardous substances with care, using proper containment, storage, and disposal methods to minimize risks.
Equipment Maintenance and Usage
Proper maintenance of laboratory tools and equipment ensures not only their longevity but also their accurate performance. Regular inspection, cleaning, and calibration of equipment should be part of standard laboratory practice. Below is a table with some tips for maintaining key laboratory tools:
| Equipment | Maintenance Tips | Proper Usage |
|---|---|---|
| Glassware | Inspect for cracks and clean thoroughly after each use. | Handle carefully to avoid breakage and contamination. |
| Pipettes | Calibrate regularly and ensure they are free of residue. | Use for precise volume measurements according to instructions. |
| Bunsen Burner | Check for gas leaks before use and clean the nozzle regularly. | Adjust the flame size according to the experiment’s requirements. |
Reviewing Acid-Base Chemistry

Understanding the interactions between acids and bases is essential for mastering key concepts in the study of reactions and equilibria. The behavior of these substances and their ability to donate or accept protons underpins many chemical processes. Whether in laboratory experiments or natural systems, grasping the fundamentals of acid-base properties and their calculations is critical to solving a wide range of problems.
Core Concepts to Focus On
To strengthen your understanding, it’s crucial to grasp the following foundational ideas:
- Acid and Base Behavior: Acids are substances that release protons (H+), while bases accept them. This proton transfer is central to their interactions and reactions.
- pH and pOH Scales: pH measures the acidity or alkalinity of a solution based on hydrogen ion concentration, with values ranging from 0 (most acidic) to 14 (most basic). pOH is similar but measures hydroxide ions.
- Strength of Acids and Bases: Strong acids and bases completely dissociate in water, whereas weak acids and bases only partially dissociate, which affects their behavior in solution.
Key Reactions and Processes
Familiarity with the following reactions will be crucial for solving problems involving acids and bases:
- Neutralization Reactions: When an acid reacts with a base, they neutralize each other to form water and a salt. These reactions are common in both lab settings and biological processes.
- Buffer Systems: Buffers help maintain a stable pH in solutions by resisting changes in acidity or alkalinity. They play a crucial role in regulating pH in living organisms and industrial processes.
- Titration Methods: Acid-base titrations involve adding a known quantity of acid to a base (or vice versa) to determine the unknown concentration of one substance. The reaction’s endpoint is typically identified through indicators or calculated based on volume and concentration.
Useful Equations
Several important equations are commonly used in acid-base calculations. Familiarizing yourself with these will help you solve related problems efficiently:
- pH = −log[H+]: This equation calculates the pH of a solution based on the concentration of hydrogen ions.
- pOH = −log[OH−]: Similar to the pH formula, this calculates the pOH based on the concentration of hydroxide ions.
- Kw = [H+][OH−] = 1 × 10−14 (at 25°C): This equation defines the ion product constant for water, used to relate the concentrations of hydrogen and hydroxide ions in pure water or neutral solutions.
Mastering these principles and equations will enable you to confidently solve problems and apply your knowledge of acids and bases in various contexts.
How to Memorize Chemical Formulas

Memorizing formulas is a critical skill for anyone studying reactions and molecular structures. The process involves learning the symbols for various elements and how they combine to form compounds. With the right techniques, you can enhance your ability to recall these formulas quickly and accurately when needed. Here are some effective strategies to help you remember them more efficiently.
Useful Techniques for Retention
Try incorporating the following methods to improve your formula memorization:
- Use Mnemonics: Creating memorable phrases or sentences from the formula’s components can help retain the structure. For example, “Cows Often Milk Fresh Grass” could help remember the formula for glucose (C6H12O6).
- Practice with Flashcards: Write formulas on one side and their corresponding names on the other. Testing yourself regularly can help reinforce your memory and highlight areas that need more focus.
- Break It Down: Rather than memorizing long formulas in one go, break them into smaller, manageable parts. Focus on memorizing one component at a time, such as the individual elements or functional groups.
Practice with Examples
Here’s a table with some common compounds and their formulas. Repetition and practice with these examples will help solidify your memory:
| Compound | Formula |
|---|---|
| Water | H2O |
| Carbon Dioxide | CO2 |
| Ammonia | NH3 |
| Sodium Chloride | NaCl |
| Glucose | C6H12O6 |
By regularly revisiting these formulas, using mnemonic devices, and employing active recall methods, you can significantly improve your ability to memorize and retain chemical formulas for any application.
Preparing for Multiple Choice Questions
Multiple choice questions test your ability to recall information, apply concepts, and think critically in a short amount of time. With the right preparation strategies, you can approach these questions with confidence. The key is to practice, review key concepts, and refine your test-taking strategies. Below are some helpful tips for preparing effectively for this type of assessment.
Effective Strategies for Multiple Choice Questions
- Understand the Question Structure: Multiple choice questions are often designed with distractors, or incorrect answers, that may seem tempting. Carefully read each option and eliminate those that are clearly wrong before focusing on the remaining choices.
- Know Key Concepts: Focus on the core principles and ideas of the subject matter. These are often the basis for questions, so being comfortable with the fundamental concepts increases your chances of choosing the right answer.
- Practice with Sample Questions: One of the best ways to prepare is by working through practice questions. This will help you familiarize yourself with the format, timing, and types of questions you may encounter.
- Watch for Key Phrases: Certain words, such as “always,” “never,” “most,” or “least,” can indicate the best answer. Pay attention to these cues to help you make better decisions.
- Time Management: During the test, allocate time wisely. If you encounter a difficult question, don’t dwell on it for too long. Move on and return to it later if time allows.
Review and Practice Regularly
To further enhance your preparation, it’s important to review your notes regularly. Repetition reinforces your memory, so take time to go over key concepts frequently. Additionally, practice with different sources of questions, such as online quizzes, study guides, or textbooks, to expose yourself to various question types and difficulty levels.
By using these strategies, you will develop the skills needed to tackle multiple choice questions efficiently and confidently.