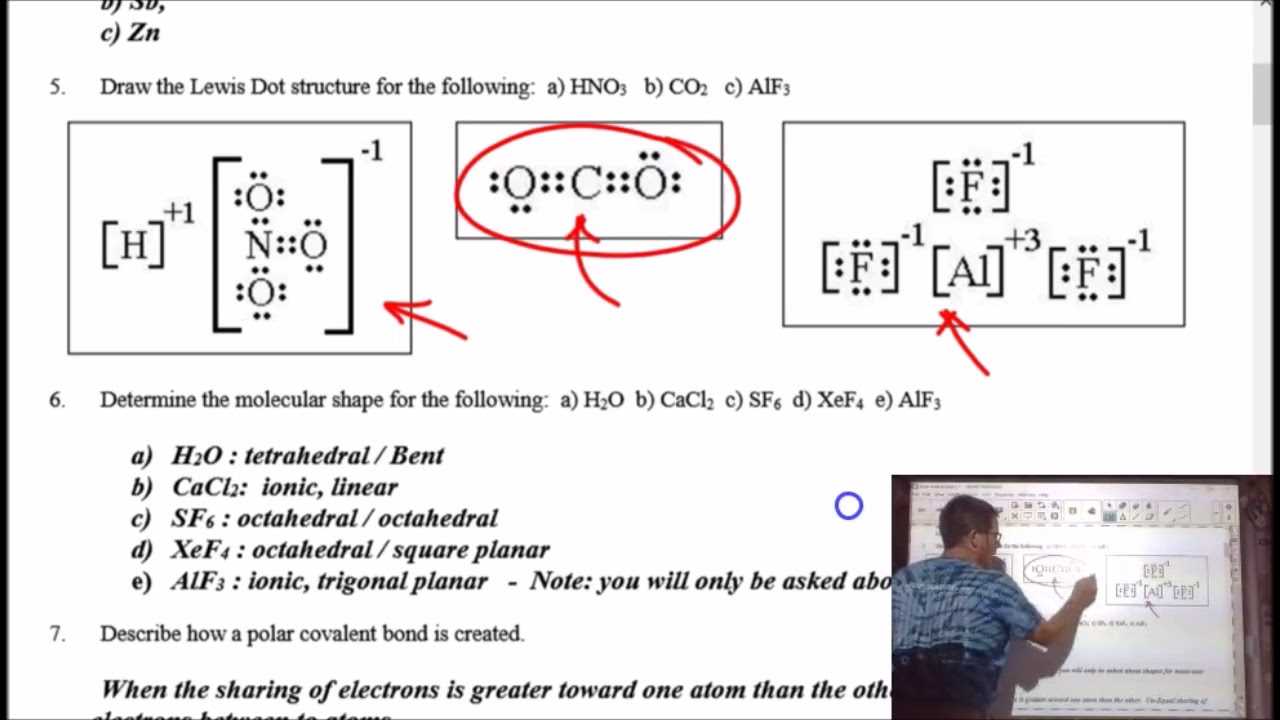
As you approach the final stage of your academic term, it’s essential to consolidate the knowledge you’ve gained throughout the course. Effective preparation is the key to demonstrating your understanding and achieving the results you aim for. This section provides valuable insights and practical advice to guide you through the process of revision and ensure you’re ready for the upcoming assessments.
In this guide, you’ll find a range of strategies to help you focus on the most critical topics, refine your skills, and tackle the most common challenges. From fundamental principles to complex problem-solving techniques, each aspect of your studies has been designed to help you succeed. With careful planning and efficient study habits, you can confidently approach the final evaluation with the tools you need to excel.
End-of-Term Preparation Guide
As the conclusion of the academic term approaches, it’s crucial to focus on consolidating your understanding and reinforcing key concepts. This section will highlight the essential topics to review and provide a structured approach to revising the material. By following this guide, you’ll ensure a thorough grasp of critical subject matter, increasing your confidence and readiness for the upcoming assessment.
Key Areas to Focus On

Focusing on the right topics is vital for efficient revision. Make sure to prioritize the core principles and areas that carry significant weight. Here are the most important subjects to revisit:
| Topic | Description |
|---|---|
| Reaction Mechanisms | Understand the fundamental processes that govern how substances interact and transform during reactions. |
| Atomic Theory | Review the structure of atoms, the periodic table trends, and how atomic models have evolved over time. |
| Organic Compounds | Familiarize yourself with the various types of organic molecules and their reactions, especially in practical contexts. |
| Thermodynamics | Focus on energy changes, enthalpy, entropy, and the laws that govern physical and chemical processes. |
Effective Revision Strategies
To maximize your study time, consider these techniques for efficient preparation:
- Practice with Past Questions: Reviewing previous assessments will help familiarize you with the question formats and the types of problems commonly encountered.
- Break Down Complex Topics: If you find a subject challenging, break it down into smaller, manageable parts and study them step-by-step.
- Group Study: Discussing topics with peers can help reinforce your understanding and offer different perspectives on difficult concepts.
Key Topics to Focus On
When preparing for the upcoming assessment, it’s crucial to focus on the areas that will have the greatest impact on your performance. By dedicating time to mastering the core subjects, you’ll be better equipped to tackle a wide range of questions. This section outlines the main topics that should be prioritized during your study sessions.
Fundamental Concepts
Understanding the foundational principles is essential for solving complex problems. Make sure you have a strong grasp of the following areas:
- Atomic Structure: Review the structure of atoms, subatomic particles, and how they influence chemical behavior.
- Reaction Types: Focus on different reaction mechanisms, including synthesis, decomposition, and displacement reactions.
- Stoichiometry: Understand the relationships between reactants and products in chemical equations and practice mole-to-mole conversions.
Advanced Topics
Once the basics are well-established, delve into more complex material. These topics are frequently tested and require a deeper level of understanding:
- Organic Chemistry: Study the structure, properties, and reactions of organic molecules, particularly hydrocarbons and functional groups.
- Thermodynamics: Grasp the principles of energy changes, heat transfer, and how they relate to the spontaneity of reactions.
- Kinetics: Review factors that affect the rate of chemical reactions, including temperature, concentration, and catalysts.
Understanding Chemical Reactions and Equations

Grasping the principles behind how substances interact and transform during reactions is essential for solving various problems. This section will focus on how to properly understand and balance equations, as well as the different types of reactions you might encounter. A strong foundation in this area will allow you to approach complex scenarios with confidence.
Types of Reactions
There are several categories of reactions, each with its unique characteristics. Be sure to familiarize yourself with the following types:
- Synthesis Reactions: Two or more reactants combine to form a single product.
- Decomposition Reactions: A single compound breaks down into two or more products.
- Single Displacement: One element displaces another in a compound.
- Double Displacement: The exchange of ions between two compounds to form new products.
- Combustion: A substance reacts with oxygen to produce energy, typically forming water and carbon dioxide.
Balancing Chemical Equations
For a reaction to follow the law of conservation of mass, the number of atoms of each element must be the same on both sides of the equation. Here are the steps to balance an equation:
- Write the unbalanced equation: List the reactants and products based on the reaction type.
- Balance the atoms: Start with elements that appear in only one reactant and one product.
- Adjust coefficients: Use coefficients to balance the number of atoms on each side, never change subscripts.
- Verify the equation: Check that all atoms are balanced and the equation is simplified to its lowest whole-number ratio.
Mastering Stoichiometry for Success
Understanding how to quantify the relationships between reactants and products is a vital skill for tackling many challenges. Stoichiometry allows you to calculate the exact amounts of substances required in a reaction, ensuring precision in problem-solving. This section will provide the tools and techniques needed to master this concept and apply it confidently in various scenarios.
Key Concepts in Stoichiometry
To approach stoichiometric calculations with accuracy, it’s essential to grasp these foundational principles:
- Conversion Factors: Use molar ratios derived from balanced equations to convert between different units such as moles, grams, and liters.
- Mole-to-Mole Ratios: Understand the relationship between the amount of one substance and the amount of another in a balanced chemical equation.
- Limiting Reactants: Identify which reactant will be completely consumed first, limiting the amount of product that can be formed.
- Theoretical Yield: Calculate the maximum amount of product that can be obtained based on the limiting reactant.
Solving Stoichiometric Problems
When solving stoichiometry problems, follow a structured approach to ensure that all steps are covered:
- Write the balanced equation: Ensure the equation is balanced before starting any calculations.
- Identify the given information: Determine what information is provided (such as mass, volume, or moles) and what needs to be calculated.
- Use conversion factors: Apply the appropriate molar ratios or unit conversions to solve for the unknown quantity.
- Check units: Make sure that all units cancel out correctly and that the answer makes sense in terms of the problem context.
Exploring Atomic Structure in Detail
Understanding the fundamental building blocks of matter is essential for grasping the behavior of substances. By exploring the components and organization of atoms, you gain insight into the properties and interactions of elements. This section will guide you through the structure of atoms, helping you understand how they contribute to chemical behavior and reactions.
Basic Components of an Atom
An atom consists of three primary subatomic particles, each with distinct characteristics. Knowing their roles is key to understanding how atoms interact:
- Protons: Positively charged particles located in the nucleus. The number of protons defines the atomic number of an element.
- Neutrons: Neutral particles found in the nucleus alongside protons. Neutrons contribute to the atom’s mass but do not affect its charge.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells. Electrons play a key role in bonding and reactions.
Electron Configuration and Energy Levels
The arrangement of electrons around the nucleus determines many of an atom’s properties. These are grouped into energy levels, each capable of holding a specific number of electrons:
- First Energy Level: Can hold up to 2 electrons.
- Second Energy Level: Can hold up to 8 electrons.
- Third Energy Level: Can hold up to 18 electrons, and so on.
The distribution of electrons across these levels is critical for determining an element’s reactivity, as atoms tend to form bonds to achieve a stable electron configuration.
Important Organic Chemistry Concepts
Organic compounds form the backbone of various biological processes and industrial applications. Understanding the essential principles that govern these molecules helps to explain their behavior and reactions. This section covers key concepts that are fundamental to mastering organic substances and their transformations in different environments.
Functional Groups and Their Significance
Functional groups are specific arrangements of atoms within a molecule that define its chemical properties. Identifying these groups is crucial for predicting the reactivity and behavior of organic molecules. Some of the most common functional groups include:
- Hydroxyl Group (-OH): Found in alcohols, it influences solubility and polarity, making it vital in hydrogen bonding and aqueous reactions.
- Carboxyl Group (-COOH): Characteristic of acids, this group is responsible for donating protons and enhancing acidity.
- Amino Group (-NH2): Present in amines and amino acids, it plays a crucial role in basic reactions and protein formation.
- Carbonyl Group (–C=O): Found in aldehydes and ketones, it is involved in various reactions, including nucleophilic addition and condensation.
Common Reaction Types
Organic molecules can undergo several reaction types, each following a distinct mechanism. Understanding these reactions is critical for predicting the behavior of molecules in different scenarios:
- Substitution Reactions: A functional group or atom in a molecule is replaced by another, often leading to the formation of new compounds.
- Elimination Reactions: Involve the removal of atoms or groups, typically resulting in the formation of a double or triple bond.
- Addition Reactions: Occur when atoms or groups are added to a molecule, particularly to unsaturated bonds like those in alkenes or alkynes.
- Rearrangement Reactions: These involve the reorganization of atoms within a molecule, forming an isomeric structure.
Acids and Bases: Key Definitions
Understanding the behavior of certain substances in solution is essential for explaining their interactions and properties. This section delves into the foundational definitions and characteristics of two important types of compounds: substances that donate protons and those that accept them. These definitions help clarify how various substances behave in aqueous environments and their reactions with one another.
Acids are substances that can donate a proton (H+) to another molecule. Their strength is often determined by how readily they release these protons. A strong acid completely dissociates in water, while a weak acid only partially dissociates. Acids are often characterized by their sour taste and ability to change the color of indicators such as litmus paper, turning it red.
Bases are substances that can accept protons or donate hydroxide ions (OH−) in solution. They tend to have a bitter taste and slippery texture. Bases are essential in neutralizing acids, and like acids, they can also be classified as strong or weak based on their ability to dissociate in water. Strong bases dissociate completely, whereas weak bases dissociate only partially. In the presence of an indicator, bases typically turn litmus paper blue.
These two types of compounds play crucial roles in various chemical reactions, such as neutralization, where an acid and a base combine to form water and a salt. Understanding these concepts provides a foundation for exploring their more complex behaviors and applications in different contexts.
Balancing Chemical Equations Effectively
Understanding how to balance reactions is a fundamental skill in analyzing chemical processes. The goal is to ensure that the number of atoms of each element is the same on both sides of the equation, reflecting the conservation of mass. This section will guide you through the steps necessary to balance equations efficiently and accurately.
To balance a chemical equation, follow these steps:
- Step 1: Write down the unbalanced equation with correct formulas for each reactant and product.
- Step 2: Count the number of atoms of each element on both sides of the equation.
- Step 3: Start by balancing the atoms that appear in only one reactant and one product.
- Step 4: Adjust coefficients for other elements, keeping in mind the need to preserve whole-number values.
- Step 5: Double-check the balance of all elements before finalizing the equation.
Here’s an example of a balanced equation:
| Unbalanced Equation | Balanced Equation |
|---|---|
| H2 + O2 → H2O | 2H2 + O2 → 2H2O |
Notice how the number of hydrogen and oxygen atoms is the same on both sides in the balanced equation. Practice with different reactions to gain a better understanding and improve your balancing skills.
Thermodynamics in Assessments

Understanding the principles that govern energy changes during chemical reactions is crucial for solving problems in various scientific contexts. This section focuses on how to approach the study and application of thermodynamic concepts, including the laws of energy conservation, enthalpy, entropy, and Gibbs free energy, which frequently appear in problem-solving scenarios.
Thermodynamic principles are essential for predicting whether a reaction will occur spontaneously and for calculating the energy required or released during a process. Key concepts to master include:
- First Law of Thermodynamics: The total energy of a system is conserved, meaning energy cannot be created or destroyed, only transferred or converted.
- Enthalpy (ΔH): The heat content of a system at constant pressure, which is used to determine the heat absorbed or released during a reaction.
- Entropy (ΔS): A measure of the disorder or randomness in a system, important for understanding the spontaneity of a process.
- Gibbs Free Energy (ΔG): A function that combines enthalpy and entropy to predict the direction of a reaction; if ΔG is negative, the reaction is spontaneous.
These concepts are integral to solving thermodynamic problems, and practicing their application can significantly improve understanding and performance in related assessments.
Understanding Chemical Kinetics
The study of how reactions progress over time and the factors that influence their rates is an essential aspect of many scientific processes. This section delves into the principles behind reaction rates, providing a deeper understanding of how and why chemical processes occur at different speeds under varying conditions.
Reaction rates are affected by several factors, including temperature, concentration, and the presence of catalysts. To understand and predict these rates, it is important to familiarize oneself with key concepts such as:
- Rate of Reaction: The change in concentration of reactants or products over time. It can be influenced by various factors, such as temperature and pressure.
- Activation Energy: The minimum energy required for a reaction to occur. Lower activation energy means faster reactions.
- Concentration and Rate: A higher concentration of reactants typically leads to a faster reaction rate due to more frequent collisions between particles.
- Catalysts: Substances that increase the rate of a reaction without being consumed in the process by lowering the activation energy.
Additionally, understanding reaction order and mechanisms can help predict the behavior of reactions more accurately. The rate law and the collision theory provide frameworks for quantifying how various factors influence the speed of chemical processes.
Electrochemistry and Its Applications
Understanding the relationship between electricity and chemical reactions is a fundamental area of study with numerous practical applications. This field explores how chemical reactions can produce electrical energy and how electricity can drive chemical changes, leading to innovations in energy storage, batteries, and industrial processes.
Electrochemical processes are central to several technologies, from powering devices to synthesizing materials. Some key concepts to explore include:
- Redox Reactions: These involve the transfer of electrons between substances, playing a crucial role in both natural and industrial processes.
- Electrolysis: The process of using electrical energy to drive a non-spontaneous chemical reaction, such as the decomposition of water into hydrogen and oxygen.
- Galvanic Cells: Devices that convert chemical energy into electrical energy through spontaneous redox reactions, commonly used in batteries.
- Fuel Cells: A type of galvanic cell that generates electricity from the reaction of a fuel (often hydrogen) with oxygen, offering a clean energy source.
These processes not only have significant scientific implications but also impact industries such as energy production, electronics, and manufacturing. By mastering the principles of electrochemistry, you can better understand and harness the power of electricity in practical and innovative ways.
The Periodic Table: Trends and Patterns
The organization of elements within a table reveals clear patterns in their properties, which can be understood through trends in atomic structure, reactivity, and behavior. These patterns provide valuable insight into the relationships between elements and allow scientists to predict their interactions and characteristics based on their position in the table.
Several key trends emerge across periods and groups, offering a framework for understanding element behavior. Some important patterns to consider include:
- Atomic Radius: The size of an atom tends to decrease across a period from left to right due to increasing nuclear charge, while it increases down a group as additional electron shells are added.
- Ionization Energy: The energy required to remove an electron from an atom increases across a period and decreases down a group. This trend is related to the effective nuclear charge and atomic radius.
- Electronegativity: The tendency of an atom to attract electrons in a chemical bond increases across a period and decreases down a group, with fluorine being the most electronegative element.
- Metallic vs. Non-metallic Properties: Elements on the left side of the table are typically metals with higher conductivity and malleability, while elements on the right side are non-metals with distinct properties.
Periodic Trends in Reactivity
Understanding how reactivity changes across the table is essential for predicting the behavior of elements in chemical reactions. For instance, alkali metals exhibit increasing reactivity down the group, while halogens display decreasing reactivity as you move down.
Utilizing Periodic Trends in Predictions
These trends and patterns not only help in identifying elements but also allow for the prediction of their reactions, helping to guide experimental processes and applications in various scientific fields.
Reviewing Moles and Molecule Calculations
In scientific calculations, understanding how to work with quantities at both microscopic and macroscopic levels is fundamental. By converting between moles, mass, and particles, it’s possible to quantify reactions, predict products, and analyze substances. This section focuses on essential methods for calculating the amounts of substances involved in chemical processes, utilizing basic principles that apply universally to matter.
Key Concepts and Conversions
To navigate these calculations, it’s important to grasp the following key principles:
- Avogadro’s Number: The constant 6.022 × 1023 defines the number of particles in one mole of a substance. It allows for the conversion between moles and individual molecules, atoms, or ions.
- Molar Mass: This is the mass of one mole of a substance, typically expressed in grams per mole. It is calculated by adding the atomic masses of all the elements in a compound.
- Mass-Mole Relationship: To convert between the mass of a substance and the number of moles, use the formula:
Number of Moles = Mass (g) ÷ Molar Mass (g/mol).
Similarly, to find mass from moles, the formula is:
Mass (g) = Number of Moles × Molar Mass (g/mol).
- Particle Calculations: The number of molecules or atoms in a sample can be determined by multiplying the number of moles by Avogadro’s number.
Number of Particles = Moles × 6.022 × 1023.
Applications in Calculations
These concepts are critical when working with chemical reactions. For example, they are used to determine the amount of reactants required, the product yield, or the volume of gases at standard conditions. Mastering these conversions is essential for precise measurements and predictions in experiments and real-world applications.
Tips for Effective Time Management
Managing your time efficiently is essential to achieving success in any academic or professional task. By organizing your activities, setting clear goals, and maintaining discipline, you can enhance productivity and reduce stress. The following strategies will help you make the most of your available time while avoiding procrastination and feeling overwhelmed.
Key Strategies for Staying Organized
- Develop a Clear Plan: Break down large tasks into smaller, manageable chunks. Assign deadlines for each task and create a daily or weekly schedule to guide your work.
- Set Priorities: Focus on the most important tasks first, especially those with approaching deadlines. Prioritize based on urgency and complexity.
- Avoid Distractions: Identify common distractions (social media, excessive noise) and create an environment conducive to focused work. Consider turning off notifications or using apps to block distractions.
- Take Regular Breaks: Prevent burnout by scheduling short breaks to recharge. Use techniques like the Pomodoro method, which involves alternating between work and breaks at set intervals.
Tools to Help You Stay on Track
Using time management tools can make it easier to stick to your plan and monitor your progress. The following table presents some of the most effective tools for staying organized:
| Tool | Purpose |
|---|---|
| Task Management Apps | Apps like Todoist or Trello help organize tasks and track progress, making it easier to stay on top of deadlines. |
| Digital Calendars | Google Calendar or Outlook can help schedule your time, set reminders, and allow you to visualize your commitments. |
| Time Tracking Apps | Tools like Toggl track how much time you spend on specific tasks, providing insight into your productivity. |
| Traditional Planners | Some people prefer writing down tasks and goals in physical planners or bullet journals to stay organized. |
By applying these tips and utilizing effective tools, you can enhance your productivity, manage your workload efficiently, and reduce stress, leading to better outcomes in your tasks and projects.
Common Mistakes to Avoid During Exams
Many individuals fall into certain pitfalls when faced with a high-pressure evaluation. By being aware of common errors, you can avoid distractions and ensure you make the best use of your time and knowledge. Preparation, focus, and careful execution play key roles in achieving success during these assessments.
Key Mistakes to Steer Clear Of
- Skipping Instructions: Not carefully reading the instructions can lead to misunderstanding the requirements of the task. Always ensure you fully understand the question before starting to answer.
- Poor Time Management: Underestimating the time needed for each section or question can result in incomplete answers. Allocate time wisely based on the complexity and marks for each part.
- Overlooking Simple Errors: Small calculation mistakes or spelling errors can cost valuable points. Double-check your work before submitting, especially when dealing with numbers or specific terminology.
- Rushing Through the Test: In the rush to finish, you may overlook questions or details that are crucial. Take your time to think carefully and avoid making hasty decisions.
How to Avoid These Mistakes
- Practice Under Time Constraints: Simulate real testing conditions during your study sessions to become more comfortable with the pace and stress.
- Stay Calm and Focused: Avoid panicking if a question seems difficult. Move on to easier questions first, then come back to the challenging ones later when you’ve had time to think.
- Review Your Work: If time permits, review your answers for any overlooked mistakes or unclear explanations. Small corrections can make a big difference.
- Prepare Thoroughly: A well-prepared individual is less likely to feel rushed or uncertain. Make sure your study sessions are organized and focused on understanding key concepts.
By being mindful of these common mistakes and implementing strategies to avoid them, you can approach your evaluation with confidence and maximize your performance.
Strategies for Answering Multiple Choice Questions
Approaching multiple choice questions effectively requires a combination of careful reading, critical thinking, and strategic elimination. By staying focused and using a systematic method to evaluate the options, you can increase your chances of selecting the correct answer. Each question may contain subtle clues that, when identified, lead to the best solution.
Approach Each Question with Precision
- Read the Question Carefully: Take time to understand what the question is asking before looking at the choices. Pay attention to key terms or qualifiers like “always,” “never,” or “most likely” that may help guide your decision.
- Eliminate Clearly Incorrect Options: Start by identifying the answers that are obviously wrong. This reduces the number of choices and gives you a better chance of picking the right one from the remaining options.
- Evaluate Each Choice: After eliminating the obvious incorrect answers, compare the remaining options. Consider the pros and cons of each and ask yourself which is most aligned with what the question is seeking.
Additional Techniques for Success
- Don’t Rush Your Decision: Take your time to think through each question. Rushing through the choices may lead to overlooked details or rash conclusions.
- Trust Your First Instinct: If you feel confident in your first answer, don’t second-guess yourself. Often, your initial reaction is the right one, unless you find concrete evidence to the contrary.
- Look for Answer Patterns: Sometimes, multiple choice questions follow patterns, such as repeating certain words or ideas across answers. While not always reliable, recognizing these patterns can help you make a more informed decision.
By applying these strategies, you can approach multiple choice questions with greater confidence and increase your accuracy in selecting the correct answers.
Practicing Past Exam Questions for Preparation
Working through previous test questions is one of the most effective methods to enhance your readiness for an assessment. By engaging with questions from past assessments, you familiarize yourself with the format, types of questions, and areas frequently covered. This practice not only reinforces your knowledge but also improves your ability to recall and apply concepts under pressure.
Benefits of Using Past Questions
- Improves Time Management: Practicing with old questions allows you to simulate the real test environment. By timing yourself while answering, you can learn to manage your time efficiently, ensuring you don’t spend too long on any single question.
- Identifies Weak Areas: Going through past questions helps highlight areas where you may be less confident or knowledgeable. This allows you to target your revision efforts on topics that need the most attention.
- Boosts Confidence: Repeated exposure to the question format and content will help you feel more comfortable when facing similar questions. This builds confidence, reduces anxiety, and enhances overall performance.
Effective Strategies for Practicing
- Review Answers in Detail: Simply completing the questions is not enough. After answering, thoroughly review your responses to understand any mistakes or gaps in knowledge. Analyze why certain answers were incorrect and correct your understanding.
- Simulate Real Conditions: When practicing, try to replicate actual test conditions by removing distractions and adhering to the time limits. This gives you a more realistic feel of the test, helping you build endurance and stay focused.
- Work with Study Groups: Collaborating with peers can provide diverse perspectives on the questions. Discussing difficult questions with others helps you approach problems from different angles and enhances learning.
Consistently practicing past questions is a powerful tool for improving both your knowledge and test-taking skills, leading to more successful outcomes.