
As you approach your final evaluations, it is crucial to focus on mastering the essential concepts that will be tested. By organizing your study materials and revisiting key ideas, you can ensure a thorough understanding of the subject matter. This approach will help you feel more confident and prepared for the challenges ahead.
During this period, emphasizing core principles and refining your problem-solving techniques will make a significant difference. It is important to understand how each topic connects to others, forming a cohesive framework of knowledge. With proper planning and practice, you can maximize your chances of success and tackle even the most challenging questions with ease.
Effective preparation is not just about reviewing textbooks but also about applying your knowledge to real-world scenarios. Focus on mastering both theoretical concepts and practical applications to achieve the best results.
Chemistry Semester Exam Review Plan
When preparing for your final assessments, creating a structured plan is essential for effective revision. The key to success lies in organizing your study time and focusing on the most critical topics. This approach will allow you to systematically cover all necessary material while preventing you from feeling overwhelmed.
Setting Clear Goals and Priorities
Start by identifying the areas that require the most attention. Some topics may need more focus than others, depending on your strengths and weaknesses. Prioritize these areas, and allocate more time to them during your study sessions. This method will ensure that you make the most out of your preparation time.
Time Management and Practice
Effective time management is vital for covering all necessary material. Break your study sessions into manageable blocks, leaving time for breaks and review. Incorporate practice problems and mock scenarios to strengthen your understanding and improve problem-solving skills. The more you practice, the more confident you’ll feel on test day.
Key Concepts to Focus On
Focusing on the most important principles is essential for a successful outcome. While there may be a wide range of topics, some concepts are more fundamental and will be featured more prominently in the assessment. Identifying these key areas and reviewing them thoroughly will provide a solid foundation for tackling any question that comes your way.
Foundational Concepts
Start by revisiting the core ideas that form the backbone of the subject. These concepts often serve as the building blocks for more advanced topics. Here are a few crucial areas to concentrate on:
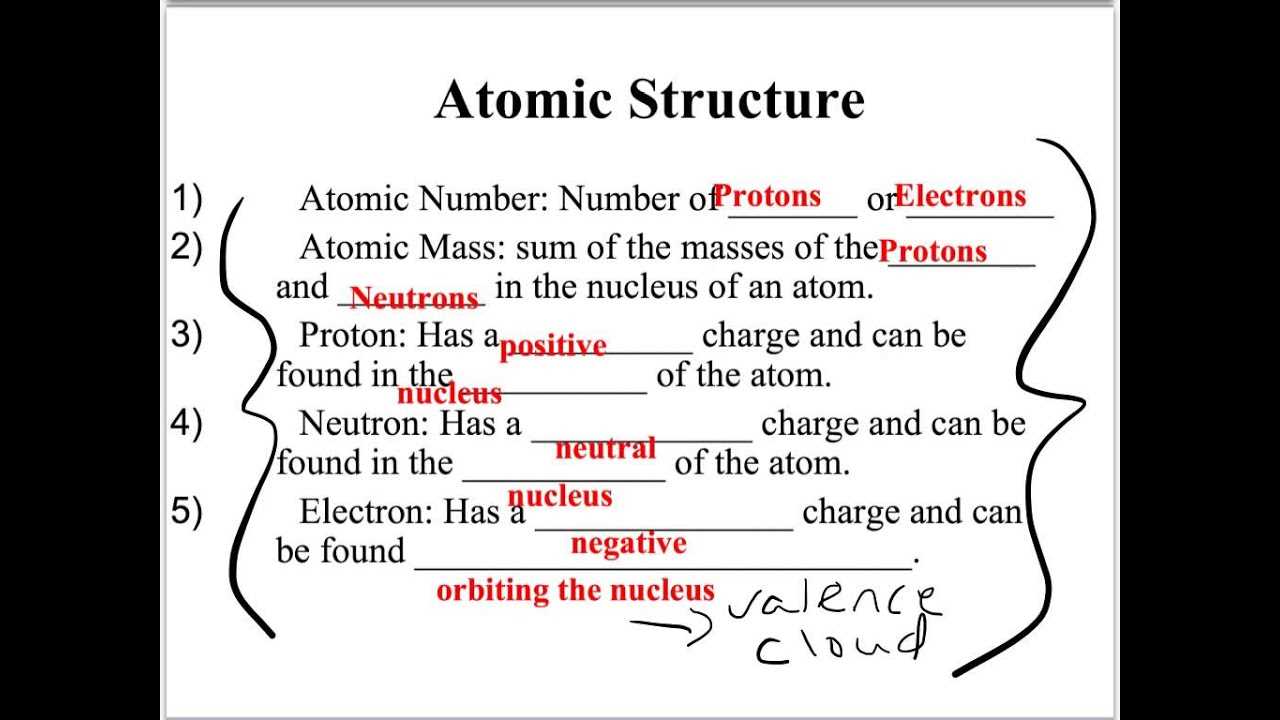
- Understanding atomic structure and bonding
- Mastering stoichiometry and its applications
- Revising the laws of thermodynamics
- Grasping the principles of equilibrium and kinetics
Advanced Topics to Strengthen
In addition to the foundational knowledge, it’s important to refine your understanding of more complex ideas. These topics often appear in higher-level questions, so dedicating extra time to them will improve your overall performance. Consider focusing on:
- Acid-base reactions and pH calculations
- Redox reactions and electrochemistry
- Organic compounds and reaction mechanisms
- Coordination chemistry and complex ions
Understanding Chemical Reactions Basics
Mastering the fundamentals of chemical processes is key to solving many of the challenges you’ll encounter. Whether you’re balancing equations or predicting reaction outcomes, having a clear understanding of how substances interact will make a significant difference. Grasping the basics provides the foundation for tackling more complex topics later on.
Types of Reactions
There are several types of reactions that frequently appear in assessments. Knowing how to recognize and categorize them is essential for understanding how different compounds react under various conditions. Some of the most common reaction types include:
- Synthesis reactions: Combining two or more substances to form a new compound
- Decomposition reactions: Breaking down a compound into simpler substances
- Single displacement reactions: One element replaces another in a compound
- Double displacement reactions: Two compounds exchange components to form new substances
- Combustion reactions: A substance reacts with oxygen, often producing energy in the form of heat and light
Balancing Chemical Equations
Balancing equations is a critical skill for solving reaction problems. It ensures that the law of conservation of mass is upheld, meaning the number of atoms of each element remains constant throughout the reaction. To balance equations, focus on adjusting coefficients, not the subscripts of molecules. Start with elements that appear in the least number of compounds, and proceed step by step to ensure that both sides of the equation are equal in terms of mass and charge.
Important Lab Techniques for Success

Proficiency in laboratory techniques plays a critical role in accurately conducting experiments and obtaining reliable results. Mastering these skills ensures that you can perform tasks efficiently, minimize errors, and interpret data with confidence. Whether you are preparing solutions or handling instruments, being precise and methodical is key to your success.
Proper Handling of Equipment
Knowing how to correctly use and maintain laboratory equipment is essential for reliable results. Whether it’s a balance, burette, or thermometer, understanding the purpose of each tool and how to handle it properly prevents contamination and ensures accurate measurements. Always calibrate instruments before use and follow the manufacturer’s instructions to ensure accuracy.
Accurate Measurement Techniques
Precision in measuring substances is critical for experiments where the exact quantity of reagents matters. Techniques like titration, pipetting, and volumetric analysis require a steady hand and attention to detail. When measuring liquids, always read the meniscus at eye level and ensure that measurements are consistent. For solid substances, weigh them carefully and avoid contamination by using clean glassware and equipment.
How to Master Stoichiometry
Understanding how substances interact in a chemical process and determining the quantities involved is crucial for accurate problem-solving. Stoichiometry, a key concept in this area, involves using relationships between reactants and products to predict the outcomes of reactions. By mastering this skill, you will be able to tackle various types of questions with ease.
Steps to Approach Stoichiometric Problems
When working with stoichiometry, following a structured approach is essential. Break the problem down into manageable steps to ensure accuracy:
- Write a balanced equation: Ensure the reaction is balanced with correct stoichiometric coefficients.
- Convert units to moles: Use molar mass to convert grams or molecules into moles.
- Apply mole ratios: Use the coefficients from the balanced equation to convert between reactants and products.
- Convert moles back to desired units: Use the molar mass or other conversion factors to find the required quantities.
Common Pitfalls to Avoid

While stoichiometry may seem straightforward, certain errors can lead to incorrect results. Watch out for these common mistakes:
- Forgetting to balance the equation before starting calculations.
- Misinterpreting mole ratios and using incorrect conversion factors.
- Skipping unit conversions or rounding too early in the process.
Tips for Memorizing Periodic Table
Mastering the arrangement of elements is essential for understanding how they interact and behave in various reactions. The periodic table is a valuable reference that organizes elements by their properties and atomic structure. However, memorizing all the elements and their properties can be challenging. By using the right techniques, you can make the learning process more manageable and efficient.
Break It Down Into Sections
Instead of attempting to memorize the entire table at once, divide it into smaller, more manageable parts. Focus on one section at a time, such as:
- Alkali metals
- Halogens
- Noble gases
- Transition metals
Learn each group’s properties and trends before moving on to the next section. This approach will help you understand the relationships between elements, making it easier to remember their positions.
Use Mnemonics and Visual Aids
Mnemonics are powerful tools for recalling complex information. Create phrases or stories that associate the elements with their symbols or positions in the table. Visual aids, such as color-coded charts or flashcards, can also help reinforce your memory. The more senses you engage, the stronger your recall will be.
Strategies for Organic Chemistry Questions
Organic reactions can be particularly challenging due to their complexity and the wide range of possible compounds and mechanisms. To succeed, it’s important to adopt strategies that will help you break down problems into simpler components. Understanding the underlying principles, practicing regularly, and recognizing patterns are key to answering questions accurately.
Understand Functional Groups and Their Reactions
Familiarize yourself with the common functional groups and the types of reactions they undergo. Each group has distinct reactivity patterns that you can apply to predict the products of a reaction. Focus on:
- Recognizing functional groups such as alcohols, aldehydes, ketones, and carboxylic acids
- Understanding how these groups behave in nucleophilic substitutions, eliminations, and additions
- Memorizing typical reaction mechanisms for each class of compounds
Practice Reaction Mechanisms
Understanding reaction mechanisms is critical for solving organic questions. Rather than just memorizing reactions, focus on learning the steps involved in each mechanism. This will allow you to predict outcomes and draw out detailed reaction pathways. Start by practicing simple mechanisms, such as:
- SN1 and SN2 substitutions
- E1 and E2 eliminations
- Electrophilic additions to alkenes
By consistently practicing and understanding these mechanisms, you will improve your ability to approach more complex organic problems with confidence.
Balancing Equations with Ease
Balancing chemical equations is a fundamental skill that ensures the law of conservation of mass is upheld. It involves adjusting the coefficients of reactants and products so that the same number of atoms of each element are present on both sides of the equation. This process is essential for accurately representing chemical reactions and predicting outcomes.
To balance equations effectively, start by identifying the elements involved and counting the number of atoms of each element on both sides. Begin with elements that appear in the least number of compounds, and adjust their coefficients accordingly. Once the less complex elements are balanced, move on to the more complex ones. In some cases, balancing oxygen or hydrogen atoms may require a bit more attention, but with practice, this becomes easier.
Keep in mind that you should never change the subscripts in a chemical formula to balance an equation. Only the coefficients can be adjusted. Regular practice with different types of reactions, such as synthesis, decomposition, and combustion, will help you build confidence and master this essential skill.
Understanding Acids and Bases
Acids and bases are fundamental concepts in understanding how substances interact in various reactions. These compounds play key roles in numerous processes, from neutralization reactions to the regulation of pH levels in biological systems. Understanding their properties, behavior, and how they react with each other is essential for mastering many concepts.
Acids typically release hydrogen ions (H⁺) when dissolved in water, making the solution acidic. Bases, on the other hand, release hydroxide ions (OH⁻), resulting in an alkaline solution. The strength of an acid or base is determined by its ability to dissociate in water, which can be quantified by the pH scale ranging from 0 to 14. A pH below 7 indicates an acid, while a pH above 7 indicates a base.
To understand the behavior of acids and bases, it is crucial to recognize their roles in neutralization reactions, where an acid and a base react to form water and a salt. Mastering these concepts allows you to predict the outcome of such reactions and apply them in real-world scenarios, such as titrations and buffer solutions.
Critical Equilibrium Principles to Review
Equilibrium refers to the state in which the rates of the forward and reverse reactions are equal, leading to no net change in the concentration of reactants and products. Understanding the principles that govern equilibrium is essential for predicting the behavior of systems under various conditions. Mastering these concepts will enhance your ability to solve related problems and predict how changes in conditions affect the system.
Le Chatelier’s Principle
This principle explains how a system at equilibrium responds to changes in concentration, pressure, or temperature. The system will shift in such a way that it counteracts the disturbance. Key points include:
- Concentration changes: Adding or removing reactants/products shifts the equilibrium to the side that reduces the disturbance.
- Pressure changes: In gaseous systems, increasing pressure shifts the equilibrium toward the side with fewer gas molecules.
- Temperature changes: Heat acts as a reactant or product depending on whether the reaction is exothermic or endothermic.
Equilibrium Constant (K)
The equilibrium constant is a numerical value that expresses the ratio of concentrations of products to reactants at equilibrium. Understanding how to interpret and calculate the equilibrium constant is vital for solving equilibrium-related problems. Keep in mind:
- K > 1: The system favors the formation of products.
- K : The system favors the reactants.
- K = 1: The system is equally balanced between reactants and products.
Explaining Thermodynamics in Chemistry
Thermodynamics plays a crucial role in understanding how energy flows and transforms during chemical processes. It explores the principles that govern heat transfer, work, and the direction of reactions. By mastering these concepts, you can predict whether a reaction will occur spontaneously and understand the energy changes involved.
At the core of thermodynamics are the concepts of energy conservation and entropy. Energy, which cannot be created or destroyed, is transformed from one form to another, such as from heat to work. Entropy measures the disorder or randomness of a system, and it tends to increase over time, dictating the spontaneity of reactions. A key relationship in thermodynamics is the Gibbs free energy, which combines enthalpy and entropy to predict the direction of a process. A negative value for Gibbs free energy indicates that the reaction is spontaneous.
To further grasp these ideas, understanding the laws of thermodynamics is essential. The first law, which is essentially the law of energy conservation, states that the total energy in a system is constant. The second law highlights the tendency of systems to move toward increased disorder, while the third law describes how entropy approaches a minimum value as temperature approaches absolute zero. These principles provide a framework for analyzing a wide variety of systems and reactions.
Top Resources for Revision
When preparing for assessments, it’s essential to use a variety of tools and materials to reinforce your understanding and strengthen your skills. Utilizing diverse resources will help you grasp complex topics more effectively and ensure you are well-prepared for any questions that may arise. From textbooks to online platforms, each resource serves a unique purpose in enhancing your knowledge and boosting your confidence.
Here are some of the best resources to aid in your preparation:
| Resource | Type | Purpose |
|---|---|---|
| Textbooks | Printed or digital | In-depth coverage of topics, explanations, and practice questions. |
| Online Learning Platforms | Websites or apps | Interactive lessons, quizzes, and video tutorials for visual learners. |
| Flashcards | Physical or digital | Quick recall and memorization of key terms and formulas. |
| Practice Papers | Past papers or mock tests | Familiarize with question formats and improve time management. |
| Study Groups | Peer-led | Collaborative learning, sharing notes, and discussing difficult concepts. |
By integrating these resources into your study plan, you can enhance both your theoretical and practical understanding of the subject, making your revision process more effective and efficient.
Practice Problems for Better Retention
One of the most effective ways to solidify your understanding and ensure long-term retention of material is through consistent practice. By actively engaging with problem-solving tasks, you reinforce key concepts and sharpen your analytical skills. Working through a variety of problems helps to identify weak areas, boosts confidence, and prepares you for similar questions in assessments.
Below is a table of different types of practice problems that can aid in your study process:
| Problem Type | Description | Benefit |
|---|---|---|
| Conceptual Questions | Questions that test your understanding of core principles. | Improves comprehension and ensures solid grasp of foundational topics. |
| Calculation Problems | Mathematical problems that require numerical solutions. | Strengthens problem-solving skills and familiarity with equations and formulas. |
| Scenario-Based Questions | Situational problems that apply theories to real-world examples. | Helps apply knowledge to practical situations and enhances critical thinking. |
| Multiple-Choice Questions | Questions with a set of options where only one is correct. | Tests quick recall and the ability to discern the most appropriate answer. |
| Short Answer Questions | Questions that require brief, focused responses. | Develops concise expression and sharpens written communication skills. |
Incorporating these different problem types into your study routine will help you strengthen your knowledge and improve retention. Regular practice, especially under timed conditions, mimics real testing scenarios and boosts your ability to recall information quickly and accurately.
Effective Time Management for Assessments
Mastering time management is a crucial skill when preparing for any assessment. The ability to allocate sufficient time for each subject, prioritize tasks, and maintain focus can significantly reduce stress and improve performance. Planning your study schedule in advance, while also leaving room for breaks and rest, ensures that you can cover all necessary material without feeling overwhelmed.
Here are some strategies to optimize your time and enhance your productivity:
- Create a Study Schedule: Outline daily and weekly study goals to help you stay on track. Allocate more time to challenging topics while ensuring regular review of easier subjects.
- Set Priorities: Tackle the most important or difficult topics first when your mind is freshest. This way, you can make the most of your productive hours.
- Break Tasks into Manageable Chunks: Avoid cramming by breaking larger topics into smaller sections. This makes studying feel less daunting and helps retain information better.
- Use Time Blocks: Study in focused blocks of time, such as 25–30 minutes of concentrated work followed by a 5–10 minute break. This approach, known as the Pomodoro technique, helps maintain focus and prevent burnout.
- Track Your Progress: Regularly assess your progress to ensure you’re meeting your study goals. If needed, adjust your schedule to allocate more time to weaker areas.
- Avoid Procrastination: Begin your study routine early to avoid last-minute stress. Consistent effort over time yields better results than cramming the night before.
By implementing these time management techniques, you can maximize your preparation time and enter your assessment with confidence and composure. Prioritizing smart study habits and effective time allocation leads to a more organized and successful approach to achieving your goals.
Common Mistakes to Avoid During Assessments
During an assessment, it’s easy to fall into common traps that can negatively impact your performance. These mistakes often stem from poor planning, lack of preparation, or simple carelessness. Recognizing and addressing these pitfalls ahead of time can help you approach the test with confidence and clarity.
Here are some key mistakes to avoid:
- Skipping Instructions: Not reading the instructions carefully can lead to missed details or errors. Always take a moment to review the guidelines before starting.
- Rushing Through Questions: It’s tempting to speed through a test to finish quickly, but rushing can lead to careless mistakes. Take your time to read each question thoroughly.
- Overlooking Easy Questions: Don’t assume simple questions are too basic to make mistakes on. Sometimes, the easiest questions carry the most weight and can help boost your score.
- Not Managing Time: Failing to allocate enough time to each section can result in incomplete answers. Prioritize more difficult questions but ensure you leave time for everything.
- Leaving Questions Blank: If unsure about an answer, don’t leave it blank. Often, partial credit is given for attempted responses, so it’s better to write something rather than nothing.
- Second-Guessing Yourself: Overthinking your answers can lead to unnecessary changes. Stick to your first instinct unless you’re sure you made an error.
- Not Reviewing Your Work: If time permits, always review your answers before submitting. Look for any missed details, arithmetic errors, or incomplete thoughts.
- Skipping Breaks: Taking short breaks can help clear your mind and refresh you, improving your focus. Don’t forget to pause if you feel your attention waning.
By avoiding these common mistakes, you can improve your test-taking strategy and increase your chances of success. Careful planning, mindful pacing, and focused attention can make a significant difference in your performance.
Preparing for Multiple Choice Questions
Multiple choice questions often appear in assessments and require a specific approach to tackle efficiently. They may seem straightforward at first, but there are strategies that can help you answer them correctly and avoid common mistakes. By preparing properly and using effective techniques, you can boost your accuracy and confidence during this section.
Here are some tips for handling multiple choice questions:
- Understand the Question: Carefully read each question and ensure you understand what it’s asking before looking at the answer options. This helps eliminate irrelevant choices.
- Eliminate Obvious Incorrect Answers: Often, some options will be clearly wrong. Cross them out to narrow down your choices and improve your odds of selecting the correct answer.
- Look for Keywords: Pay attention to keywords in the question and options, such as “always,” “never,” or “most likely.” These can give you clues about the correct answer.
- Don’t Overthink: Trust your first instinct unless you’re sure you’ve made an error. Overanalyzing can lead to confusion and second-guessing.
- Watch for Traps: Some questions include “distractor” answers designed to mislead you. Be cautious of options that sound familiar but don’t directly answer the question.
- Check for Absolutes: Words like “always” or “never” can indicate extreme answers that are often incorrect, while more moderate terms like “usually” or “sometimes” can be more likely to be true.
- Use Process of Elimination: If you’re unsure, eliminating one or more incorrect answers can increase your chances of choosing the right option from the remaining choices.
- Review Your Answers: If time allows, go back and check your answers, especially for questions you were unsure about. Look for any mistakes you might have missed initially.
With these strategies, you can approach multiple choice questions with greater precision and increase your likelihood of success. Practice these techniques to feel more prepared and confident during assessments.
How to Stay Calm During the Test
Staying calm during a high-pressure situation like an assessment can be challenging, but it’s essential for optimal performance. Stress can cloud your thinking and lead to mistakes, so developing strategies to manage anxiety and maintain focus is crucial. By practicing relaxation techniques and staying organized, you can approach the test with a clear mind and a steady hand.
Here are some practical tips to help you remain composed:
- Practice Deep Breathing: Take slow, deep breaths to help reduce your heart rate and calm your nerves. Inhale deeply for four seconds, hold for four seconds, and exhale for four seconds.
- Stay Positive: Replace negative thoughts with positive affirmations. Remind yourself that you have prepared and that you are capable of succeeding.
- Break the Test into Manageable Parts: Instead of focusing on the entire test, tackle one section at a time. This will make the task feel more manageable and help reduce feelings of overwhelm.
- Stay Hydrated and Take Breaks: Drink water to stay refreshed, and if permitted, take short breaks to stretch or relax for a moment before continuing.
- Visualize Success: Picture yourself calmly working through the questions and succeeding. Visualization can be a powerful tool in boosting confidence and reducing stress.
- Focus on What You Can Control: Accept that you cannot change the difficulty of the questions, but you can control your approach and effort. Focus on doing your best rather than worrying about the outcome.
Remember that staying calm is a skill that can be developed with practice. By implementing these strategies, you will be better equipped to handle the stress of the situation and perform at your best.