
Mastering the core principles of science is crucial for achieving success in any academic field. As you prepare for your upcoming assessment, understanding key topics and honing your problem-solving skills will provide you with the confidence needed to tackle challenging questions. Whether you’re focusing on basic concepts or complex calculations, effective preparation is the key to excelling.
Conceptual clarity is fundamental to solving questions accurately and efficiently. Reviewing vital material in an organized manner ensures that you can apply your knowledge when needed. In addition to theory, practicing with example problems strengthens your ability to think critically and react swiftly during the test.
Throughout this guide, we will break down essential topics and provide clear explanations, so you can approach the test with a solid understanding. With the right strategies, you will be well-equipped to handle all types of questions and demonstrate your mastery of the subject matter.
Key Concepts for Effective Preparation
To succeed in your upcoming assessment, it’s essential to focus on understanding the critical principles and methods that are central to the material. A solid grasp of the key topics will not only help you recall information but also enable you to apply it efficiently during the test. Below are the core areas to focus on as you prepare.
- Atomic Structure – Understanding the basic building blocks of matter, atomic models, and electron configuration.
- Chemical Reactions – Be familiar with different types of reactions, reaction mechanisms, and how to balance chemical equations.
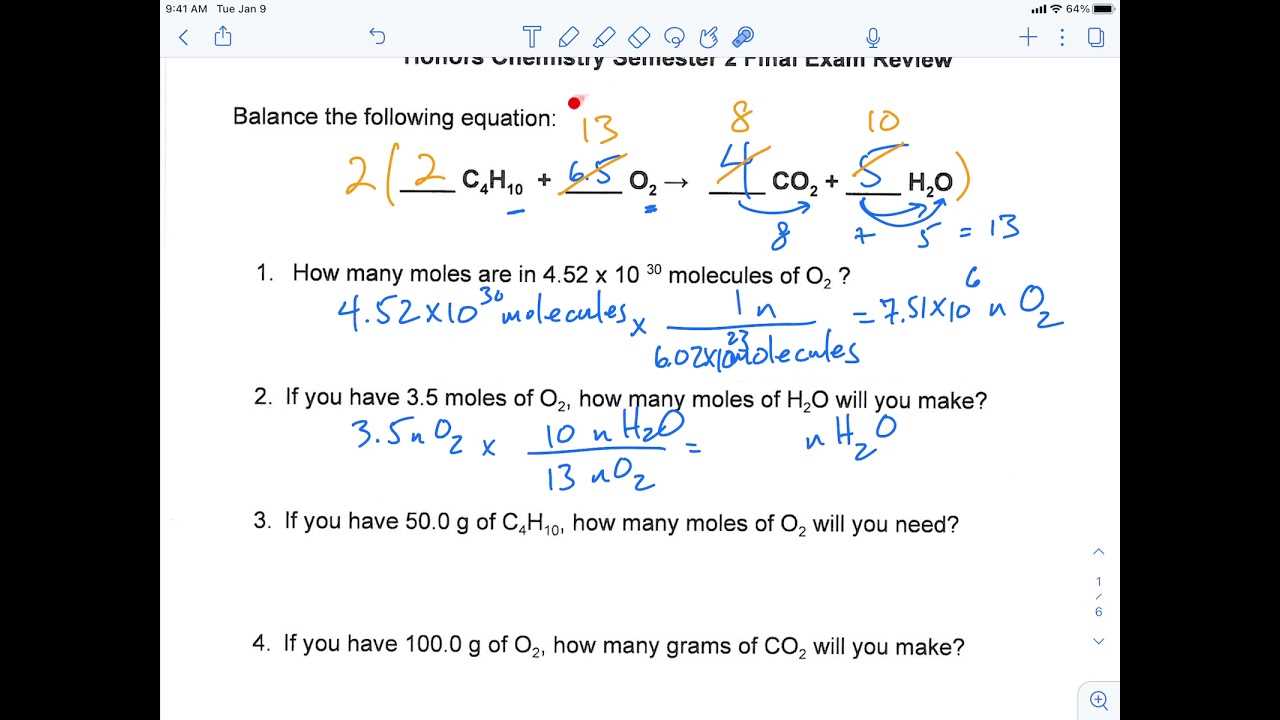
- Stoichiometry – Master the calculation of reactants and products in chemical reactions, using mole ratios and conversions.
- Thermodynamics – Grasp the concepts of heat, work, and energy changes during chemical processes.
- Periodic Table Trends – Know the organization of elements and the trends in atomic size, electronegativity, and ionization energy.
- Acids and Bases – Understand the properties, theories, and calculation of pH, pOH, and concentrations.
- Gas Laws – Familiarize yourself with the relationships between pressure, volume, and temperature in gases.
- Solution Chemistry – Focus on solubility, concentration, and dilution calculations for solutions.
Each of these topics plays a vital role in mastering the subject matter. Ensure that you practice problems related to these areas to gain the proficiency needed to approach the test with confidence.
By focusing on these core topics, reviewing essential formulas, and applying your knowledge to practice problems, you’ll be well-equipped to perform well under test conditions. Keep your study sessions structured, and don’t forget to allocate time for thorough revision of key concepts to reinforce your understanding.
Key Concepts to Review for the Test
When preparing for an assessment, it’s crucial to focus on the fundamental principles that underpin the subject. A deep understanding of these core ideas will not only help you recall information but also allow you to apply it effectively under pressure. The following concepts are essential for success and should be thoroughly understood before the test.
Start with the basics of matter and its properties, and build upon this foundation by diving into the different types of reactions. A solid understanding of molecular behavior and atomic interactions will guide you through more complex topics such as reaction rates and equilibrium. Additionally, concepts like energy changes in reactions, the behavior of gases, and solution concentrations are pivotal for tackling a wide range of questions.
It’s also important to practice solving problems related to each concept, as applying theoretical knowledge to practical scenarios is often a key component of assessments. Focusing on these critical areas will ensure that you are well-prepared for the challenges the test may present.
Chemical Reactions and Equations Explained
Understanding how substances interact and transform is central to the study of matter. Reactions occur when different compounds or elements combine or break apart to form new substances. Each transformation can be represented through a chemical equation, which details the substances involved and how they change during the process.
In any reaction, it’s important to identify the reactants (the starting materials) and the products (the substances formed). These equations help visualize the rearrangement of atoms, ensuring that mass is conserved. Balancing these equations is key to understanding the stoichiometry of a reaction, as it allows you to determine the proportions in which substances react and are produced.
Additionally, different types of reactions–such as synthesis, decomposition, single replacement, and double replacement–require specific strategies for interpretation. Mastering these concepts is crucial for solving problems related to reactions and predicting the outcomes of various chemical processes.
Understanding Atomic Structure and Bonding
The arrangement of particles within an atom is fundamental to understanding the behavior of matter. Atoms are composed of smaller components–protons, neutrons, and electrons–that interact in specific ways, dictating the chemical properties of elements. A clear understanding of atomic structure provides the foundation for exploring how atoms bond to form compounds.
Atomic structure refers to the organization of an atom’s nucleus, which contains protons and neutrons, surrounded by electrons. These electrons occupy different energy levels or orbitals, and their distribution influences the reactivity of the atom. The number of protons determines the element’s identity, while the number of electrons in the outermost shell, or valence electrons, plays a critical role in chemical bonding.
Bonding occurs when atoms share or transfer electrons in order to achieve stability. In covalent bonds, atoms share electrons, while ionic bonds involve the transfer of electrons from one atom to another. The nature of these bonds determines the properties of the resulting compounds, including their physical state, reactivity, and ability to conduct electricity. Understanding these interactions allows for the prediction of how substances will behave in different chemical processes.
Tips for Mastering Stoichiometry
Stoichiometry is a key skill in solving problems involving the amounts of substances in chemical reactions. It connects the amounts of reactants and products through a series of well-defined steps. By mastering stoichiometric calculations, you can determine how much of each substance is required or produced in a reaction.
To excel in stoichiometry, it’s important to focus on understanding the relationships between moles, mass, and volume. Start by converting between units using dimensional analysis, making sure to set up ratios correctly. The mole ratio from a balanced chemical equation serves as the bridge between reactants and products, allowing you to calculate quantities involved in a reaction.
Practice regularly with a variety of problems, and always double-check your units to ensure consistency. By breaking down complex problems into smaller, manageable steps and focusing on conversions, you will increase both your confidence and accuracy in solving stoichiometry problems.
Balancing Chemical Equations with Ease
Balancing chemical reactions is a fundamental skill for understanding how substances interact during transformations. A balanced equation ensures that the law of conservation of mass is followed, meaning that the number of atoms of each element remains the same on both sides of the equation. The key to mastering this skill is developing a systematic approach to ensure both sides of the reaction are equal in terms of reactants and products.
Step-by-Step Process
To balance an equation effectively, follow these steps:
- Write the unbalanced equation with all reactants and products.
- Count the number of atoms of each element on both sides.
- Adjust the coefficients (the numbers in front of molecules) to balance the atoms, starting with the most complex molecules.
- Check that the atoms are balanced for each element, and verify that the equation adheres to the law of conservation of mass.
Example of a Balanced Reaction
Consider the following reaction:
| Unbalanced Equation | Balanced Equation |
|---|---|
| H2 + O2 → H2O | 2H2 + O2 → 2H2O |
In this example, we begin by counting the number of atoms on both sides. After adjusting the coefficients (in this case, a coefficient of 2 in front of H2 and H2O), the equation is balanced, ensuring that the number of hydrogen and oxygen atoms is equal on both sides.
With consistent practice, balancing equations becomes an intuitive process, allowing for quick and accurate problem solving during any related tasks or tests.
Acids and Bases Key Definitions
Understanding the fundamental concepts of acids and bases is essential for grasping how substances interact in aqueous solutions. These two categories of compounds have distinct properties and behaviors, and their reactions are crucial in many chemical processes. The following definitions highlight key concepts that will help you understand the behavior of these substances.
Acid-Base Theories
There are several ways to classify acids and bases, depending on the theory used. Here are the most common definitions:
| Term | Definition |
|---|---|
| Acid (Arrhenius) | A substance that increases the concentration of hydrogen ions (H+) in an aqueous solution. |
| Base (Arrhenius) | A substance that increases the concentration of hydroxide ions (OH–) in an aqueous solution. |
| Bronsted-Lowry Acid | A substance that donates a proton (H+) to another substance. |
| Bronsted-Lowry Base | A substance that accepts a proton (H+) from another substance. |
| Lewis Acid | A substance that accepts an electron pair in a chemical reaction. |
| Lewis Base | A substance that donates an electron pair in a chemical reaction. |
Common Properties
Acids and bases exhibit specific behaviors that help in their identification:
- Acids tend to have a sour taste, turn blue litmus paper red, and can react with metals to release hydrogen gas.
- Bases have a bitter taste, feel slippery to the touch, and turn red litmus paper blue.
These basic properties and definitions serve as the foundation for more complex topics related to acid-base reactions, pH levels, and the role of acids and bases in various chemical systems.
How to Solve Gas Law Problems
Solving problems related to gases involves understanding the relationships between pressure, volume, temperature, and the amount of gas. These relationships are described by various gas laws that allow you to predict how gases behave under different conditions. The key to solving gas law problems is recognizing which law to apply based on the known variables and what you’re trying to find.
The most common approach is to identify the variables provided in the problem, then select the appropriate equation based on the scenario. For example, when pressure and volume are inversely related, Boyle’s Law is used. When temperature and volume are directly proportional, Charles’s Law comes into play. For more complex situations, the ideal gas law (PV = nRT) can be used to calculate unknown quantities when pressure, volume, temperature, and moles of gas are involved.
Steps to solve gas law problems:
- Identify the known values: Determine which variables are given (pressure, volume, temperature, or moles).
- Choose the correct gas law: Select the appropriate equation based on the information provided (Boyle’s Law, Charles’s Law, or Ideal Gas Law).
- Rearrange the equation: Solve for the unknown variable by algebraically rearranging the formula.
- Plug in the values: Insert the known values into the equation and calculate the result.
- Check units: Ensure that all units are consistent and properly converted, such as temperature in Kelvin and pressure in atmospheres or pascals.
By practicing different types of problems and familiarizing yourself with each gas law, you’ll be able to tackle a wide variety of scenarios with confidence and accuracy.
Periodic Table Trends You Should Know
The periodic table is more than just a chart of elements; it reveals patterns and trends that dictate the behavior and properties of different substances. Understanding these trends can help you predict how elements will interact and behave in chemical reactions. Whether you’re analyzing atomic size, electronegativity, or ionization energy, recognizing these patterns is key to mastering the subject.
Key Trends in the Periodic Table
Here are some of the most important trends to understand:
- Atomic Radius: As you move across a period (left to right), atomic size decreases due to increasing nuclear charge. As you move down a group (top to bottom), atomic size increases because additional electron shells are added.
- Ionization Energy: This is the energy required to remove an electron from an atom. Ionization energy generally increases across a period (due to stronger attraction between the nucleus and electrons) and decreases down a group (as the outermost electrons are farther from the nucleus).
- Electronegativity: Electronegativity refers to an atom’s ability to attract electrons in a bond. It increases across a period and decreases down a group. Fluorine is the most electronegative element.
- Electron Affinity: This measures the energy change when an atom gains an electron. Electron affinity tends to become more negative across a period, indicating a stronger attraction for electrons.
Understanding These Trends in Action
These trends help explain why certain elements behave in similar ways. For instance, metals generally have lower ionization energies and are more likely to lose electrons, whereas non-metals have higher ionization energies and tend to gain electrons. This knowledge helps in predicting the formation of ions and the types of chemical bonds that can form.
Familiarity with these periodic table trends can significantly improve your ability to predict and understand chemical behavior, making them essential concepts for anyone studying this field.
Significance of Moles in Chemistry
The concept of the mole is fundamental in understanding the quantities of substances involved in chemical reactions. It serves as a bridge between the atomic scale and the macroscopic world, allowing scientists to relate the number of particles (atoms, molecules, ions) to measurable quantities such as mass or volume. Without this unit, working with chemicals would be far more complex and less precise.
The Role of the Mole
At the core of many calculations in the field is the ability to count and compare the number of atoms or molecules in a substance. The mole provides a standardized way to do this, and it’s tied to the Avogadro constant, which defines one mole as exactly 6.022 × 1023 particles. Understanding this concept is essential for conducting reactions, calculating yields, and determining stoichiometric relationships.
- Molecular Quantities: The mole allows us to convert between the mass of a substance and the number of molecules or atoms it contains, simplifying complex calculations.
- Stoichiometry: This concept is essential in stoichiometric calculations, where the mole ratio between reactants and products helps predict the outcome of chemical reactions.
- Gas Volume Calculations: The mole concept also plays a key role in calculating the volume of gases under certain conditions, often utilizing the ideal gas law.
- Concentration Calculations: The mole helps in determining molarity, which is crucial for solution preparation and dilution processes.
Applications of the Mole Concept
In real-world scenarios, understanding the mole is critical for fields such as pharmaceutical development, environmental science, and industrial processes. For example, determining the correct amount of reactants to use in a reaction or calculating how much of a product can be obtained requires a solid grasp of this concept.
In conclusion, the mole is more than just a number–it’s a vital concept that underpins much of what happens in both the laboratory and the natural world. By learning how to apply this concept, you’ll be able to make sense of reactions and measurements with ease.
Solutions and Concentration Calculations
In many scientific fields, working with solutions is a common practice, whether it’s for creating mixtures, conducting experiments, or preparing reagents. Understanding how to calculate the concentration of a solution is essential for accurate and effective use of substances. Concentration refers to the amount of solute dissolved in a given volume of solvent, and mastering the calculations behind it is key to ensuring proper preparation and consistency in results.
Methods for Determining Concentration
There are several ways to express the concentration of a solution, each serving a different purpose depending on the context. Some of the most common methods include:
- Molarity (M): The most widely used method, molarity is calculated by dividing the number of moles of solute by the liters of solution. The formula is:
M = moles of solute / liters of solution. - Molality (m): Molality is another concentration measure, defined as the moles of solute per kilogram of solvent. It is particularly useful when temperature changes could affect volume measurements.
- Percent Concentration: This can be expressed as mass percent, volume percent, or weight/volume percent, depending on whether you are working with solid or liquid solutes.
- Normality (N): Normality is often used in acid-base reactions and refers to the number of equivalents of solute per liter of solution. It is particularly relevant for titrations and redox reactions.
Calculating Dilution and Concentration Adjustments
One important aspect of working with solutions is knowing how to dilute concentrated solutions to achieve a desired concentration. The dilution equation is essential for these calculations:
- Formula for Dilution:
C1V1 = C2V2, where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
This equation allows you to calculate the volume of stock solution needed to make a specific volume of a diluted solution with a given concentration.
By understanding and applying these concentration formulas, you’ll be able to prepare solutions with precise amounts of solute, ensuring consistency and accuracy in your work, whether in a laboratory setting or an industrial process.
Exploring Thermochemistry and Heat Transfer
Understanding the movement and transfer of heat is fundamental to many scientific processes. In both physical and chemical reactions, energy changes play a crucial role in determining how systems behave. Heat transfer, whether absorbed or released during a reaction, helps explain the energy dynamics involved. This area of study explores how thermal energy interacts with matter and the methods used to quantify and control it in various scenarios.
The Basics of Heat Flow
Heat is a form of energy that flows from a region of higher temperature to one of lower temperature. The transfer of heat can occur through three main mechanisms:
- Conduction: The transfer of heat through direct contact between molecules or particles, typically occurring in solids.
- Convection: The movement of heat through fluids (liquids or gases) caused by the motion of the fluid itself.
- Radiation: The transfer of heat in the form of electromagnetic waves, such as infrared radiation, which does not require a medium.
Thermochemical Reactions and Energy Changes
Thermochemistry focuses on the study of heat changes that occur during chemical reactions. These reactions either absorb heat, making them endothermic, or release heat, making them exothermic. Understanding these processes is key to predicting the outcome of many reactions and their feasibility in practical applications.
- Exothermic Reactions: Reactions that release energy to the surroundings, often as heat, causing the temperature of the surroundings to increase.
- Endothermic Reactions: Reactions that absorb energy from their surroundings, causing the temperature of the surroundings to decrease.
The heat change in a chemical reaction is often quantified using the concept of enthalpy. The enthalpy change (ΔH) indicates whether a reaction is endothermic or exothermic. In calorimetry experiments, the amount of heat released or absorbed can be measured to determine the enthalpy change of the system.
By mastering the principles of thermochemistry and heat transfer, scientists and engineers can manipulate energy changes in reactions, optimizing processes in everything from industrial production to environmental science.
Important Formulae for the Exam
Mastering key equations is essential for solving a variety of problems in scientific studies. Whether dealing with reaction rates, energy changes, or concentration calculations, being familiar with the right formulae allows for quick and accurate problem-solving. This section highlights some of the most important and commonly used equations that will help you tackle a range of topics effectively.
Here are some of the crucial formulae to remember:
- Ideal Gas Law: PV = nRT
Used to calculate the properties of an ideal gas, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin. - Boyle’s Law: P1V1 = P2V2
Describes the inverse relationship between pressure and volume of a gas at constant temperature. - Charles’s Law: V1/T1 = V2/T2
Shows the direct proportionality between volume and temperature for a gas at constant pressure. - Concentration (Molarity): M = moles of solute / liters of solution
Used to find the concentration of a solution, expressed in moles per liter (M). - Energy Change in Chemical Reactions (Enthalpy): ΔH = H(products) – H(reactants)
Represents the change in enthalpy during a reaction, indicating whether the reaction is exothermic or endothermic. - Density Formula: ρ = mass / volume
Helps determine the density of a substance, with ρ representing density, mass in grams, and volume in liters or cubic centimeters. - Percent Composition: % = (mass of element / mass of compound) × 100
Used to calculate the percentage by mass of each element in a compound. - Heat Transfer Equation: q = mcΔT
Used to calculate the heat energy q absorbed or released by a substance, where m is mass, c is specific heat capacity, and ΔT is the change in temperature.
Familiarizing yourself with these formulas is vital for success. Practice using them in a variety of problems to improve both your understanding and speed when applying them during assessments.
Strategies for Tackling Multiple-Choice Questions
Multiple-choice questions can be tricky, but with the right strategies, you can maximize your chances of choosing the correct answer. These questions often contain subtle clues that, when identified, can help you eliminate incorrect options and focus on the best choice. In this section, we’ll explore effective techniques for approaching these types of questions and improving your accuracy.
1. Read the Question Carefully
It’s easy to misinterpret a question when you’re rushing, so take your time to read it thoroughly. Pay attention to keywords that define what is being asked, such as “most likely,” “least,” “except,” or “which of the following.” Understanding the question is the first step toward finding the correct answer.
2. Eliminate Clearly Wrong Answers

One of the quickest ways to narrow down your options is to eliminate answers that are clearly incorrect. Look for choices that are obviously irrelevant to the question or make no logical sense. This increases the odds of selecting the right one by reducing the number of choices.
- Look for extreme terms: Words like “always,” “never,” and “only” are often too absolute and may not apply in all cases.
- Watch for contradictions: If an answer choice contradicts other information in the question, it’s likely incorrect.
3. Use Your Knowledge to Find Clues

Sometimes, even if you’re not sure about the exact answer, you can use your general knowledge to pick up on key facts or clues. Try to think through the material logically and use your understanding of concepts to assess which answer seems most likely. Often, questions are designed with hints based on the subject matter you’ve studied.
4. Don’t Overthink It
If you’re stuck between two or more choices, trust your instincts. The first answer that comes to mind is often the correct one, unless you can identify a strong reason to change your choice. Overanalyzing can lead to confusion and doubt, so be confident in your initial judgment.
5. Time Management
Keep an eye on the clock to ensure that you don’t spend too much time on any one question. If you’re unsure, make your best guess and move on. You can always return to difficult questions later if time allows. Prioritizing the easier questions first can help build confidence and conserve time for more challenging ones.
6. Review Your Answers
If time permits, review your answers before submitting your response. Check for any mistakes you might have missed, such as misread questions or accidental selections. A second pass can often reveal overlooked details or errors in judgment.
By applying these strategies, you’ll be better equipped to approach multiple-choice questions with a clear and focused mindset. Practice regularly to build your confidence and improve your performance under timed conditions.
Preparing for Short Answer Questions
Short answer questions require you to provide more detailed responses than multiple-choice questions, often involving clear explanations, calculations, or definitions. These questions are designed to test your deeper understanding of key concepts. To perform well, it’s essential to practice concise, accurate responses that fully address what is being asked.
1. Understand the Question
Before jumping into your response, take a moment to carefully read the question. Identify the key components and what exactly is being asked. This is crucial to ensure your answer is relevant and directly addresses the requirements of the question.
2. Plan Your Answer
Organize your thoughts before writing. You don’t need a full outline, but taking a few seconds to jot down the main points will help you stay focused and cover all aspects of the question. This is especially important for questions that require multiple steps or explanations.
- Key points: Focus on the most important concepts that need to be addressed.
- Sequence: Present your answer in a logical order, especially for processes or explanations.
3. Be Clear and Concise
Avoid unnecessary details. Be precise in your explanations while ensuring that each part of your answer directly contributes to addressing the question. Clear and concise answers often score better than overly long or rambling responses.
4. Use Relevant Terminology

Whenever possible, incorporate appropriate terms and concepts from your studies. Using the correct vocabulary shows a solid understanding of the material and can help reinforce your credibility in your response.
5. Show Your Work for Calculations
If the question involves a calculation, make sure to show all the steps clearly. This allows you to earn partial credit, even if you make a mistake along the way. Always double-check your math and ensure that you’ve used the correct units and formulas.
6. Review Your Answer
If time allows, review your response before submitting. Check for any missed points, unclear explanations, or errors. A second look can often catch small mistakes or help refine your answer.
By following these strategies, you’ll be better prepared to tackle short answer questions with confidence, ensuring that your responses are well-organized, accurate, and clear. Practice is key to improving your ability to express your knowledge effectively under exam conditions.
How to Approach Lab-Based Questions
Lab-based questions often test your understanding of experimental procedures, data analysis, and the interpretation of results. These questions assess your ability to think critically and apply theoretical knowledge to practical situations. To excel in this type of question, it’s important to be systematic in your approach and to carefully consider the instructions and observations presented.
1. Understand the Experiment and Procedure
Before diving into the question, ensure that you fully understand the experiment being discussed. Revisit key details about the procedure, such as the steps involved, the materials used, and the variables controlled. This background knowledge will help you answer questions about the experiment’s design, outcomes, and conclusions more accurately.
2. Focus on the Data and Results
Data interpretation is a key part of lab-based questions. Whether it’s a table of measurements or a graph, make sure you can draw conclusions from the data provided. Look for trends, patterns, or anomalies that could be relevant to the question. Understanding how to analyze and present results will enable you to respond more effectively.
| Variable | Initial Measurement | Final Measurement | Change |
|---|---|---|---|
| Temperature | 22°C | 45°C | +23°C |
| Volume | 10 mL | 15 mL | +5 mL |
3. Address Variables and Controls
Lab-based questions may ask you to explain how different variables affect the outcome of an experiment. Be prepared to distinguish between independent variables (the ones you change) and dependent variables (the ones you measure). Additionally, identify any controls that were used to maintain the experiment’s consistency and reliability.
4. Provide Clear Explanations and Reasoning
When responding to lab-based questions, it’s important to provide clear and logical explanations. If asked to explain why a certain result occurred, refer back to the principles that govern the process, and connect your explanation to the observed outcomes. If you’re asked to suggest improvements or alternative methods, base your recommendations on sound reasoning and evidence from the experiment.
By following these guidelines, you will be able to effectively tackle lab-based questions. It’s essential to approach them with both a strong understanding of the experiment and the ability to apply that knowledge in a clear, reasoned manner. Practice and familiarity with experimental setups will help you perform well in this area.
Common Mistakes to Avoid During the Exam
While preparing for a test is crucial, how you approach the test itself can make a big difference in your performance. Avoiding common errors can help you save time and boost your confidence. Some mistakes are easy to overlook but can lead to incorrect answers or missed opportunities. Being aware of these pitfalls can improve your accuracy and overall test-taking strategy.
1. Skipping Instructions or Questions
One of the most common mistakes students make is not fully reading the instructions or questions. This can lead to misunderstanding what is being asked or overlooking specific requirements. It’s essential to read each question carefully and review any instructions related to calculations, formatting, or how to approach the answer.
2. Rushing Through the Questions
While time is a factor during any test, rushing through questions without double-checking can lead to avoidable mistakes. It’s easy to misinterpret a question or make a calculation error when you’re in a hurry. Always pace yourself and give yourself time to think before answering.
| Common Mistake | Impact | Solution |
|---|---|---|
| Skipping Instructions | Incorrect approach or missing crucial details | Read all instructions thoroughly |
| Rushing Through | Misunderstood questions or calculation errors | Take your time and review your answers |
| Overthinking Questions | Unnecessary confusion or wasting time | Stick to clear logic and basic principles |
3. Overthinking or Second-Guessing Answers
Second-guessing yourself after choosing an answer is a common mistake. It’s easy to get caught up in doubt, especially if the question seems tricky. Often, your first instinct is correct. If you find yourself constantly changing answers without a solid reason, it’s best to trust your initial judgment unless you find a clear error.
4. Not Showing Work for Calculations
When performing calculations, it’s important to show your work. Even if the answer is correct, failing to document your process can sometimes lead to losing points. Additionally, showing your work allows you to catch mistakes along the way, helping you spot errors before finalizing your answer.
Avoiding these common mistakes can significantly improve your test performance. Take your time, stay focused, and double-check your work to minimize errors. Keeping a calm and systematic approach will help you avoid pitfalls and perform to the best of your ability.
How to Effectively Manage Your Time
Time management is an essential skill, especially when preparing for important assessments or completing complex tasks. The ability to allocate your time efficiently can reduce stress and ensure that you approach each part of the test or project with focus and clarity. With the right strategy, you can maximize your productivity and avoid feeling rushed or overwhelmed.
1. Prioritize and Plan Ahead
Start by identifying the most critical tasks or topics that require your attention. Creating a clear plan with specific goals for each study session or task can help keep you on track. Allocate more time to areas that need improvement and leave time for review and practice as well. Planning ahead will also prevent the feeling of being overwhelmed and ensure that you address everything you need to in an organized manner.
2. Set Time Limits for Each Section
During the task or test, it’s easy to get caught up in one section, but this can eat up precious time. Set realistic time limits for each section or problem to ensure that you don’t spend too long on one item. When time is allocated wisely, it allows you to tackle all parts of the task with focus and efficiency. If you get stuck on a question or problem, move on and come back to it later if possible.
Effective time management allows you to complete tasks more efficiently, reduce last-minute stress, and achieve better results. By staying organized and pacing yourself, you’ll be able to handle multiple responsibilities with confidence and ease.