In this section, we explore the foundational principles behind various processes and interactions in the realm of matter. The content focuses on understanding the underlying mechanisms that drive substances to combine, transform, and produce new forms. By delving into these core ideas, we gain a deeper appreciation for how elements and compounds interact in different environments.
Critical concepts such as atomic behavior, the energy changes involved, and the factors influencing reaction rates play a crucial role in shaping how we perceive these transformations. Mastering these ideas is essential for anyone aiming to build a solid foundation in the subject, as they provide the necessary tools to navigate more complex topics.
By reviewing the key elements and their interrelationships, students can enhance their grasp of this dynamic field. The focus on clear explanations and step-by-step solutions allows for a deeper understanding of the processes that govern the natural world around us. This approach equips learners with the skills needed to apply these concepts effectively in both theoretical and practical situations.
Modern Chemistry Chapter 4 Section 1 Review Answers
This section delves into the essential principles governing various processes and transformations in the world of substances. By examining these basic concepts, we develop a clearer understanding of how elements interact with each other, leading to the formation of new compounds and reactions. The goal is to grasp the fundamental ideas that serve as the building blocks for more complex topics.
Key Concepts to Understand
- The structure and behavior of atoms in different contexts.
- The forces and energy changes involved during reactions.
- How elements and compounds interact to form new substances.
- Factors that influence reaction speed and efficiency.
Focusing on these aspects helps students better understand the mechanics behind various transformations, offering a foundation to tackle more advanced material. By strengthening knowledge in these key areas, learners can confidently proceed with more intricate problems and scenarios.
Common Challenges and Solutions
- Understanding atomic interaction can be complex. To overcome this, break down each component of the process and study how individual particles behave.
- Balancing equations and solving for unknowns often causes confusion. Practice with sample problems can improve accuracy and speed.
- Reaction rates are influenced by numerous factors such as temperature, concentration, and catalysts. Identifying these variables in specific scenarios is essential for correct predictions.
By addressing these challenges with practical strategies and clear explanations, learners can achieve a deeper understanding of the material and improve their problem-solving abilities.
Understanding Key Concepts in Chemistry

Grasping the fundamental principles behind the behavior of matter is crucial for anyone studying this field. These core ideas form the basis for understanding how substances interact, change, and form new products. By mastering these concepts, learners can navigate more complex topics and develop a comprehensive understanding of the world at the molecular level.
Atomic Structure and Interactions
The foundation of all chemical processes lies in the structure and properties of atoms. Atoms consist of a nucleus made up of protons and neutrons, surrounded by electrons. Understanding how these particles interact, bond, and share electrons is essential for predicting how substances will behave in different environments. The way atoms combine to form molecules and compounds dictates much of what happens in chemical reactions.
Energy and Reactions
Another important concept is the role of energy in chemical reactions. Every reaction involves the breaking and forming of bonds, processes that require or release energy. Whether it’s the energy needed to initiate a reaction or the energy released as products are formed, understanding how energy flows is crucial for predicting reaction outcomes and controlling processes in various applications.
How to Approach Chapter 4 Review
Successfully navigating through the material requires a clear strategy to comprehend the key topics and solve related problems. Focus on understanding the fundamental principles and their real-world applications before diving into specific examples or calculations. A systematic approach will help reinforce your grasp of the material and identify areas that need further attention.
Start by reviewing the core ideas covered in the material. Break down complex topics into smaller, more manageable sections, ensuring that you understand the underlying concepts before moving forward. Practice with sample problems and pay attention to the steps involved in solving each one. Additionally, revisiting any areas of confusion or difficulty will allow for deeper comprehension and better retention of the material.
Important Reactions Covered in Section 1
This part of the material highlights key processes that drive the transformation of substances. Understanding these reactions is essential for recognizing how elements and compounds interact to form new products under various conditions. Each reaction type plays a crucial role in the broader context of chemical processes and applications.
Combustion reactions are among the most common and involve the reaction of a substance with oxygen, often releasing energy in the form of heat and light. This type of reaction is fundamental in understanding energy production and many industrial applications.
Synthesis reactions are another important category, where two or more simpler substances combine to form a more complex product. This type of reaction is key in many synthetic processes used in manufacturing chemicals and materials.
Decomposition reactions also play a significant role, where a single compound breaks down into two or more simpler products. These reactions are critical in processes like recycling and waste management.
By familiarizing yourself with these essential reactions, you will be able to recognize their patterns and predict outcomes in various scenarios, laying a strong foundation for future learning in this field.
Key Definitions and Terms to Remember
Mastering key concepts is essential for understanding the material thoroughly. Certain terms and definitions serve as the foundation for comprehending the interactions and behaviors of substances. Familiarizing yourself with these terms not only clarifies the theoretical aspects but also enhances your ability to solve practical problems efficiently.
Core Terms to Focus On
- Element: A pure substance consisting of one type of atom, which cannot be broken down into simpler substances by chemical means.
- Compound: A substance formed when two or more elements chemically combine in fixed ratios.
- Atom: The smallest unit of matter, composed of protons, neutrons, and electrons.
- Molecule: A group of atoms bonded together, representing the smallest unit of a compound.
Key Processes and Properties
- Covalent Bond: A chemical bond formed when two atoms share one or more pairs of electrons.
- Reaction Rate: The speed at which reactants are converted into products in a chemical reaction.
- Activation Energy: The minimum amount of energy required to initiate a chemical reaction.
- Equilibrium: The state in which the rates of the forward and reverse reactions are equal, leading to no net change in concentration.
By internalizing these fundamental definitions, you will be better equipped to grasp the more complex concepts that follow and enhance your overall understanding of the subject.
Exploring Chemical Bonds and Reactions
The interaction between atoms and molecules is at the heart of all chemical processes. Chemical bonds are the forces that hold atoms together in compounds, and these bonds determine the properties of substances. By understanding how these bonds form and how they break, we can gain insight into the mechanisms behind chemical reactions. This section focuses on the types of bonds and the various ways in which substances can interact and transform.
There are two main types of chemical bonds: covalent and ionic. Each plays a distinct role in the formation of compounds, with specific properties and behaviors in different reactions. Exploring how these bonds influence the outcome of reactions helps to explain why certain substances behave in particular ways.
| Type of Bond | Key Characteristics | Examples |
|---|---|---|
| Covalent Bond | Atoms share electrons to achieve stability. Typically occurs between nonmetals. | Water (H2O), Carbon dioxide (CO2) |
| Ionic Bond | Electrons are transferred from one atom to another, creating positive and negative ions that attract each other. Occurs between metals and nonmetals. | Salt (NaCl), Magnesium chloride (MgCl2) |
| Metallic Bond | Electrons are shared freely among a lattice of metal atoms, allowing conductivity. | Copper (Cu), Iron (Fe) |
Understanding these bonds provides a solid foundation for comprehending chemical reactions. When bonds are broken and formed, energy is either absorbed or released, and this energy change is what drives reactions forward. Whether it’s the formation of new substances or the rearrangement of atoms, these processes are central to the behavior of matter in various environments.
Reviewing Atomic Structure and Elements
The fundamental units of matter are atoms, and understanding their structure is key to explaining how different substances behave and interact. The configuration of these tiny particles, along with the various elements they compose, plays a significant role in the formation of compounds and reactions. This section explores how atoms are organized and introduces the building blocks that make up the materials around us.
Structure of an Atom
An atom consists of a central nucleus, made up of positively charged protons and neutral neutrons, with negatively charged electrons orbiting around it. The number of protons in the nucleus determines the identity of the element, while the arrangement of electrons dictates how the atom will bond with other atoms. Understanding this structure is fundamental for comprehending the chemical properties of elements.
Elements and Their Role
Elements are pure substances that consist of only one type of atom. Each element has unique properties based on its atomic structure, which influences its behavior in reactions. The periodic table organizes elements based on their atomic number and other characteristics, allowing for predictions about how they will interact with other substances. This organization helps to make sense of the diverse range of elements that exist in the natural world.
Common Mistakes in Section 1 Review
As you work through the material, certain misconceptions and errors tend to occur frequently. These mistakes often arise from misunderstandings of key concepts or the misapplication of definitions and rules. Identifying these common pitfalls early can help avoid confusion and improve your grasp of the subject matter. Below are some typical mistakes to watch out for when reviewing the content.
Misunderstanding Atomic Structure
- Confusing protons and neutrons: While both particles are found in the nucleus, protons carry a positive charge, and neutrons are neutral. Misunderstanding this can lead to errors when identifying atomic numbers or mass numbers.
- Overlooking electron configuration: It’s essential to understand how electrons are arranged in different energy levels. Forgetting this can result in incorrect predictions about an atom’s behavior in bonding.
Common Errors in Bonding and Reactions
- Mixing up ionic and covalent bonds: Ionic bonds occur when electrons are transferred, while covalent bonds involve shared electrons. Confusing these two can lead to errors in understanding chemical properties and reactions.
- Forgetting about the role of energy in reactions: Energy is involved in both the formation and breaking of bonds, but many overlook its significance in driving chemical processes.
By being aware of these common mistakes and paying attention to the details, you can strengthen your understanding and avoid confusion as you continue studying the material.
Tips for Solving Chemistry Problems
Approaching problems in this field requires a clear strategy and attention to detail. Whether you are dealing with calculations, conceptual questions, or reactions, applying the right techniques can make a significant difference in finding the correct solution. Below are some effective strategies to help you tackle problems more efficiently.
| Tip | Description |
|---|---|
| Understand the Problem | Before jumping to a solution, take the time to read and analyze the question carefully. Identify the known and unknown variables to ensure you understand what is being asked. |
| Break It Down | Large or complex problems can often be simplified by breaking them into smaller steps. Work through each part methodically to avoid errors and confusion. |
| Write Down All Formulas | List relevant equations or principles before attempting the solution. Having the formulas in front of you can prevent mistakes and help you identify which one to use. |
| Check Units and Conversions | Ensure that the units used in the problem match the required units in the answer. Don’t forget to convert where necessary, as improper units can lead to incorrect solutions. |
| Practice Regularly | The more problems you solve, the more familiar you will become with common patterns and techniques. Regular practice will improve your problem-solving skills over time. |
By using these strategies and practicing consistently, you can approach problems with greater confidence and accuracy, ultimately improving your understanding of key concepts and boosting your performance in assessments.
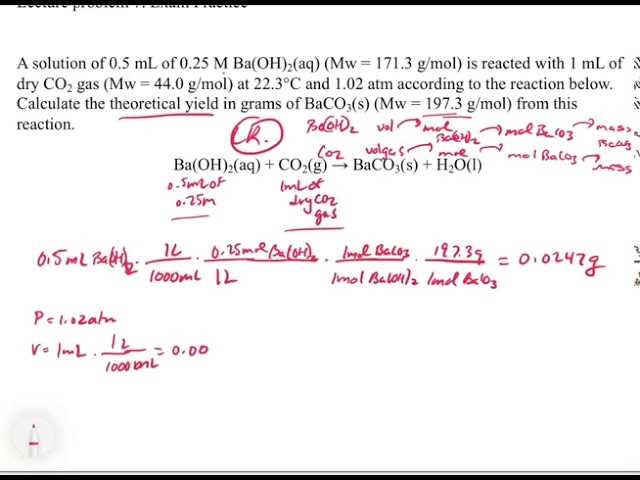
Step-by-Step Breakdown of Answers
To master the concepts discussed in this material, it is crucial to understand the process behind solving each problem. Rather than focusing solely on the final result, breaking down the steps involved helps in comprehending the underlying principles and ensures accuracy throughout the process. Below is a detailed breakdown of how to approach typical questions in this field.
First, read the question carefully to ensure that all the details are understood. Identify the key elements involved, such as the substances, quantities, or reactions that need to be considered. Once the problem is fully understood, start by organizing the given information clearly, and note down any necessary formulas or concepts that apply.
Next, determine the appropriate approach. For calculations, decide whether you need to use stoichiometry, balancing equations, or another method. For conceptual questions, make sure you are referencing the correct theories or laws that relate to the situation.
Once the strategy is clear, begin the solution process by applying the relevant equations or reasoning step by step. Keep track of all intermediate results and carefully check each calculation or assumption. If conversions or unit changes are needed, do them at this point to ensure consistency.
Finally, after arriving at the answer, double-check your work. Look over each step to verify that no details were missed or incorrectly applied. Confirm that the final result makes sense in the context of the problem and that all units and signs are correct.
Analyzing Experimental Procedures in Chemistry
Understanding the process of conducting scientific investigations is crucial for gaining insights into the behavior of matter. Every experiment involves a systematic approach to test hypotheses, observe outcomes, and draw conclusions. Analyzing the steps of an experiment helps identify potential sources of error and ensures that the results are accurate and reliable. Below are some key aspects to consider when evaluating experimental methods.
Key Considerations in Experimental Design
- Hypothesis formulation: A clear and testable hypothesis is essential for guiding the experiment. It sets the direction for the investigation and helps define the expected outcomes.
- Materials and equipment: The choice of materials and instruments can significantly affect the accuracy of results. Ensure that all tools are calibrated and appropriate for the task.
- Control variables: To isolate the effect of the independent variable, all other factors should be kept constant. This prevents unintended influences on the results.
Steps for Analyzing Results
- Data collection: Record observations and measurements carefully, ensuring precision and consistency. Accurate data is the foundation of sound conclusions.
- Data analysis: Use mathematical methods, such as calculations or statistical analysis, to interpret the collected data and identify trends or patterns.
- Identifying errors: Carefully assess any discrepancies or unexpected results. These could be due to experimental flaws, such as improper measurement or uncontrolled variables.
By following a systematic approach and carefully analyzing each phase of an experiment, researchers can draw more reliable conclusions and improve the reproducibility of their findings.
Application of Theories in Real-World Scenarios
Scientific principles provide a framework for understanding natural processes. These abstract concepts, when applied to real-world challenges, lead to practical innovations and solutions. The application of these theories can be seen across various industries, from improving manufacturing processes to developing new technologies and improving public health. Translating theoretical knowledge into action is key to solving everyday problems and enhancing the quality of life.
In many fields, such as medicine, engineering, and environmental science, the theoretical concepts learned in the classroom serve as a foundation for tackling practical issues. The following table outlines several examples of how theoretical knowledge is used to address real-world challenges.
| Industry | Real-World Application | Theoretical Concept |
|---|---|---|
| Healthcare | Drug design and delivery systems | Chemical bonding and molecular interactions |
| Manufacturing | Optimizing production lines and material properties | Thermodynamics and material science |
| Energy | Development of renewable energy sources | Electromagnetism and energy transfer |
| Environmental Protection | Pollution control and waste management | Reaction kinetics and environmental impact |
By applying scientific theories, professionals are able to innovate and create solutions that address complex issues. This bridge between theoretical knowledge and practical application is vital for progress and technological advancements.
Visual Aids for Chapter 4 Understanding
Visual representations can significantly enhance comprehension of complex concepts. By providing clear and concise images, charts, and diagrams, these tools help break down abstract ideas into more understandable forms. Whether you’re studying atomic structures, reaction mechanisms, or molecular interactions, the right visual aids offer a more intuitive grasp of the material.
Key Visual Aids for Learning
The following are some of the most effective types of visuals for deepening understanding:
- Diagrams – Visualizing molecular structures and atomic arrangements aids in grasping the relationships between different particles.
- Charts – Organizing information into tables and graphs helps highlight trends and patterns in chemical data.
- Flowcharts – These are particularly helpful for illustrating processes, such as reaction pathways or energy transformations.
- 3D Models – Interactive models provide a hands-on way to explore molecular shapes and bond angles.
Benefits of Visual Learning Tools
- Improves retention by engaging both visual and cognitive processes.
- Clarifies complex concepts through simplified, easy-to-understand representations.
- Encourages active learning, allowing students to make connections between theory and practice.
Incorporating visual aids into your study routine can make a noticeable difference in how well you understand and retain the information presented in this section. These tools not only make learning more engaging but also support a deeper, more lasting understanding of challenging topics.
Strategies for Mastering Complex Topics
Mastering difficult subjects requires a combination of focused techniques and consistent practice. Tackling complex material can often seem overwhelming, but breaking it down into manageable parts and applying effective study methods can make all the difference. Whether you’re dealing with intricate theories, detailed processes, or mathematical calculations, the right approach helps build understanding and confidence.
Effective Study Techniques
Here are some proven strategies that can aid in mastering challenging material:
- Chunking Information – Break down large amounts of data into smaller, digestible sections to prevent feeling overwhelmed.
- Active Recall – Regularly test yourself on the material to reinforce memory and identify areas that need more attention.
- Concept Mapping – Visualize the relationships between different ideas by creating diagrams that link key concepts.
- Teach What You’ve Learned – Explaining complex topics to someone else helps solidify your understanding and reveals gaps in knowledge.
Maximizing Retention and Understanding
To ensure you retain and fully understand the material, consider these additional tips:
- Practice Regularly – Frequent practice ensures familiarity with the material and helps you become comfortable with applying concepts in different scenarios.
- Use Multiple Resources – Don’t rely solely on one textbook or study guide. Explore different resources like online tutorials, videos, and interactive exercises.
- Group Study – Collaborating with peers can offer fresh perspectives, answer questions you might have, and fill in knowledge gaps.
By incorporating these strategies into your study routine, you can transform complex topics from daunting to manageable and ensure a stronger grasp of the subject. Regular practice and varied approaches will help you retain and apply the material with confidence.
Effective Study Habits for Chemistry Students
Success in challenging subjects requires more than just attending lectures and reading textbooks. It involves adopting specific habits and methods that help students absorb, retain, and apply knowledge effectively. Developing strong study techniques can lead to better understanding and improved performance, especially in subjects that require both conceptual grasp and practical application.
Key Study Strategies
To enhance learning and performance, students should consider incorporating the following strategies into their study routines:
- Active Participation in Class – Engage during lectures by taking notes, asking questions, and summarizing key points. Active involvement strengthens comprehension and retention.
- Regular Review Sessions – Consistent review helps reinforce previously learned material. Scheduling weekly sessions to revisit concepts ensures they stay fresh and can be integrated into new content.
- Practice Problem-Solving – Applying theories and concepts through practice problems strengthens understanding and reveals areas that may need more focus.
- Study in Short, Focused Intervals – Research shows that studying in 25-30 minute intervals with breaks in between helps maintain concentration and prevents burnout.
Enhancing Retention and Application
Beyond effective study methods, it is also essential to adopt practices that aid in retention and application:
- Teach and Discuss Concepts – Explaining material to others or discussing topics with peers can help solidify understanding and uncover areas of weakness.
- Utilize Visual Aids – Diagrams, charts, and flashcards can help visualize abstract concepts and make complex material easier to grasp.
- Link Theory to Real-Life Scenarios – Understanding how concepts apply to real-world situations can improve both comprehension and interest in the subject matter.
By establishing and maintaining effective study habits, students can approach their learning in a structured and productive way. With the right tools and methods, mastering difficult topics becomes a manageable and rewarding process.
How to Review Chapter 4 Efficiently
To achieve a deep understanding of complex material, it’s crucial to adopt a strategic approach when revisiting key topics. Reviewing effectively involves more than just skimming through the content; it requires organizing, reinforcing, and applying the information in a systematic way. By focusing on core concepts, utilizing active learning techniques, and managing time wisely, students can maximize their retention and understanding.
The first step in efficient review is to identify the key concepts and focus areas of the material. Instead of trying to memorize every detail, prioritize the most important ideas that are central to the subject. This can be done by reviewing outlines, summaries, and previous notes.
Next, it’s important to engage with the material actively. This can include practicing problems, creating flashcards, or discussing topics with peers. Active participation helps solidify understanding and reveals areas where more focus may be needed. Furthermore, using various methods such as diagrams or charts can help clarify abstract concepts.
Finally, setting a clear review schedule ensures that study time is optimized. Breaking the review into manageable chunks with regular breaks allows for better focus and retention. By following these steps, reviewing material becomes a more effective and efficient process, ultimately leading to a stronger grasp of the subject.