
Delving into advanced scientific subjects requires a clear understanding of foundational principles and the ability to apply them effectively. This section focuses on enhancing your analytical skills, providing clarity on intricate problems, and boosting your confidence in tackling challenging scenarios.
By exploring detailed solutions and practical examples, you’ll gain insights into patterns, mechanisms, and problem-solving strategies essential for excelling in this field. Strengthening your grasp on these concepts will not only aid in acing assessments but also lay the groundwork for future academic and professional pursuits.
The following guide is designed to help you navigate critical topics, address common pitfalls, and refine your approach. Armed with these tools, you’ll be better equipped to achieve your learning goals and excel in your studies.
Mastering Complex Assessment Challenges
Tackling advanced scientific evaluations requires not only a deep understanding of the subject matter but also strategic approaches to problem-solving. Success hinges on the ability to apply knowledge effectively, adapt to unexpected questions, and maintain focus under pressure.
To excel, it’s essential to build a robust foundation of key principles and explore how they interconnect across different scenarios. This involves recognizing patterns, analyzing detailed case studies, and understanding the logic behind various processes. Developing these skills will allow you to approach any challenge with confidence and precision.
Equally important is cultivating efficient study habits and time management techniques. Breaking down complex topics into manageable sections and prioritizing areas of difficulty can significantly enhance your preparation. Additionally, practicing under simulated conditions can help refine your speed and accuracy, ensuring you’re fully prepared to perform at your best.
Key Concepts Essential for Success
Understanding critical principles is the cornerstone of excelling in advanced scientific studies. These ideas serve as the framework for solving intricate problems, analyzing patterns, and applying logic to real-world scenarios. Building a strong grasp of these fundamentals ensures a smoother journey through challenging assessments.
Foundational Topics to Master
- Reaction mechanisms: Learn how transformations occur and the steps involved in each process.
- Structure and bonding: Understand the relationships between components and their properties.
- Energy principles: Explore concepts such as stability and transitions within systems.
- Functional groups: Recognize their roles and interactions in various contexts.
Strategies to Strengthen Understanding
- Practice consistently: Solve diverse problems to solidify your grasp of key ideas.
- Use visual aids: Diagrams and charts can simplify complex information.
- Break down challenges: Divide multifaceted questions into smaller, manageable parts.
- Engage in discussions: Collaborating with peers can provide fresh perspectives.
Mastering these essential concepts and strategies not only enhances your ability to perform well but also builds a foundation for tackling even more advanced topics in the future.
Preparing Effectively for Advanced Assessments

Thorough preparation is key to achieving success in complex scientific evaluations. A well-structured plan ensures that you cover essential material, strengthen your understanding, and approach challenges with confidence. By focusing on both foundational principles and practical application, you can maximize your performance.
Steps to Build a Strong Preparation Plan
- Review foundational theories: Begin by revisiting the core principles to solidify your understanding.
- Organize study materials: Use concise notes, summaries, and diagrams to streamline your review process.
- Set achievable goals: Break your preparation into smaller milestones to track progress effectively.
- Incorporate active learning: Solve
Preparing Effectively for Advanced Assessments
Thorough preparation is key to achieving success in complex scientific evaluations. A well-structured plan ensures that you cover essential material, strengthen your understanding, and approach challenges with confidence. By focusing on both foundational principles and practical application, you can maximize your performance.
Steps to Build a Strong Preparation Plan
- Review foundational theories: Begin by revisiting the core principles to solidify your understanding.
- Organize study materials: Use concise notes, summaries, and diagrams to streamline your review process.
- Set achievable goals: Break your preparation into smaller milestones to track progress effectively.
- Incorporate active learning: Solve practice problems and work through case studies to apply your knowledge.
Helpful Techniques for Better Retention
- Use mnemonics: Memorize complex processes and sequences more easily with creative associations.
- Create flashcards: Test your recall of key terms and definitions quickly and efficiently.
- Practice regularly: Dedicate consistent time to revisiting difficult topics and solving varied problems.
- Simulate test conditions: Attempt timed practice sessions to improve speed and accuracy.
A disciplined and strategic approach to preparation not only helps build confidence but also equips you to tackle even the most demanding assessments successfully.
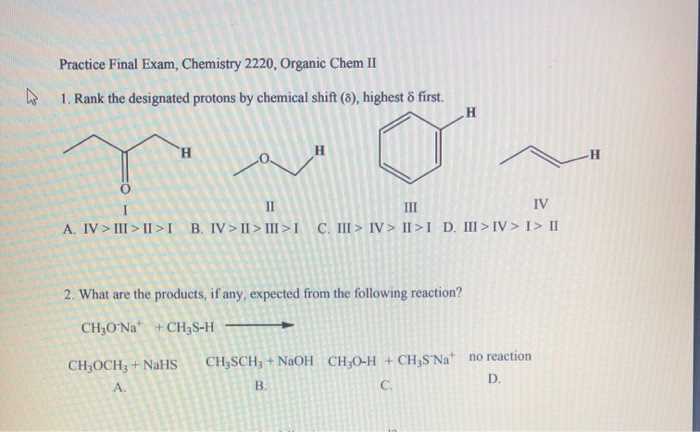
Detailed Solutions to Practice Questions
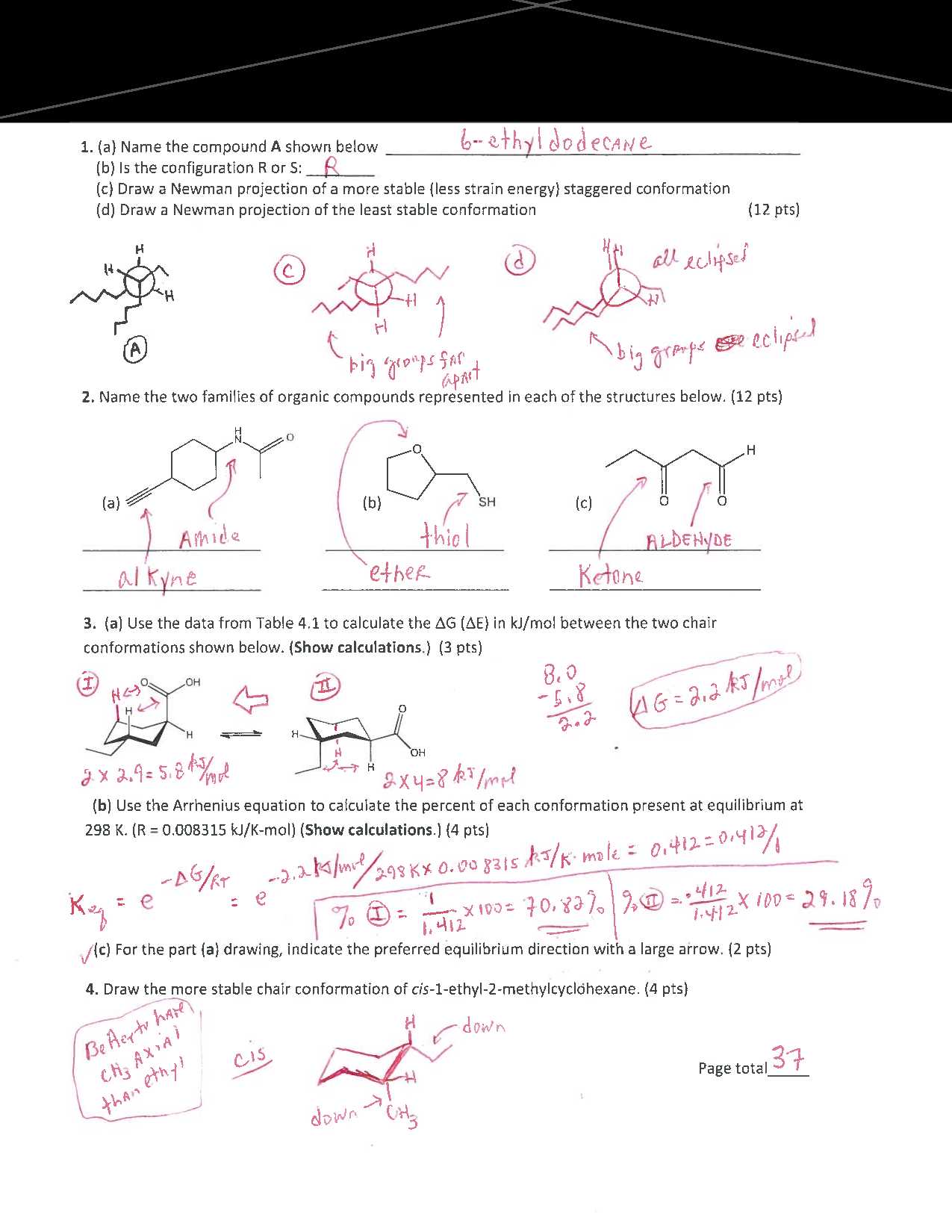
Working through sample problems with clear and thorough explanations is essential for deepening your understanding of challenging concepts. By analyzing solutions step by step, you can identify patterns, improve problem-solving strategies, and enhance your ability to approach similar tasks with confidence.
Below is a table showcasing typical problem types, common pitfalls, and detailed explanations for overcoming them:
Problem Type Common Pitfall Step-by-Step Solution Reaction Analysis Ignoring intermediate steps Break down the reaction into stages, identify reactants and products, and ensure all intermediates are accounted for. Mechanism Prediction Overlooking possible pathways Examine all potential routes, consider stability factors, and select the most plausible sequence based on principles. Structure Identification Confusing functional group properties Compare features systematically, cross-check against provided data, and verify the arrangement matches expected behavior. Energy Calculations Skipping key variables Write out all given values, use the correct formula, and double-check each calculation for consistency. Breaking down solutions in this manner not only highlights effective approaches but also emphasizes the importance of attention to detail. Consistently applying these methods will help you tackle similar challenges with greater ease and accuracy.
Breaking Down Reaction Mechanisms

Understanding the step-by-step progression of a transformation is crucial for mastering complex scientific processes. Reaction mechanisms describe how molecules interact, rearrange, and form new products through a series of intermediate steps. By analyzing each stage carefully, you can predict the outcome of reactions and identify key factors influencing the process.
Key Steps in Mechanism Analysis
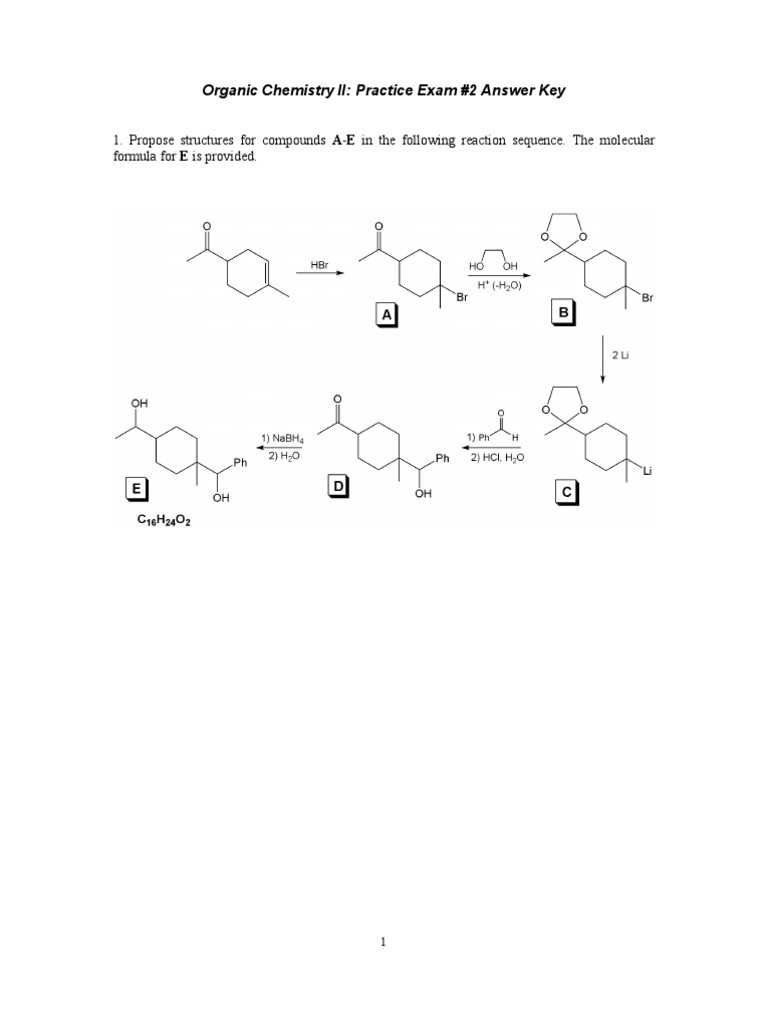
To break down a mechanism, it’s important to focus on the following stages:
- Identify reactants and products: Clearly outline the starting materials and final compounds.
- Recognize intermediates: Determine any transient species formed during the reaction.
- Understand the role of catalysts: Understand how catalysts facilitate the reaction without being consumed.
- Highlight electron movement: Use arrows to track electron shifts throughout each stage.
Common Challenges and Strategies
Many students struggle with understanding how to apply the rules governing reaction steps. A few tips for overcoming these difficulties include:
- Work backward: Start from the final product and reverse-engineer the steps to understand the process.
- Focus on reaction conditions: Take note of temperature, solvents, and other factors that influence the mechanism.
- Practice regularly: The more mechanisms you study, the more familiar and intuitive they become.
By carefully analyzing and practicing reaction mechanisms, you can build a strong foundation for solving complex problems and predicting the outcomes of various processes.
Study Techniques That Boost Retention
Mastering complex material requires more than just reading through notes. Retaining information for long-term use depends on how you engage with the content and reinforce your understanding. Effective study techniques focus on enhancing memory, improving recall, and making learning more efficient.
Some of the most effective methods for improving retention include:
- Active recall: Instead of passively reviewing notes, actively test your knowledge by recalling key points from memory.
- Spaced repetition: Revisit material at increasing intervals to reinforce learning and prevent forgetting.
- Mind mapping: Create visual diagrams to connect related concepts and see the bigger picture.
- Chunking: Break down large amounts of information into smaller, manageable pieces for easier retention.
In addition, try to teach what you learn. Explaining concepts to others can reinforce your own understanding and highlight areas that need further review. The more actively you engage with the material, the better your ability to remember and apply it in the future.
Analyzing Structures and Their Properties
Understanding the relationship between molecular structures and their physical and chemical properties is key to mastering complex scientific topics. By examining how atoms are arranged and interact within a molecule, you can predict its behavior, reactivity, and stability. This process requires a deep understanding of bonding, symmetry, and electronic configuration.
Key Factors Influencing Molecular Behavior
Several elements influence how molecules function and react, including:
- Bonding and functional groups: Different bonds and functional groups in molecules affect their reactivity and properties.
- Electron distribution: The placement and movement of electrons within a molecule determine its chemical reactivity and physical characteristics.
- Molecular shape: The 3D structure of a molecule dictates its interaction with other molecules, impacting reactions and properties.
Analyzing Through Spectroscopy and Modeling
Tools like spectroscopy allow for the visualization of molecular structures, helping to confirm hypotheses about a molecule’s composition and behavior. Modeling software can also aid in understanding how different configurations influence molecular properties. Both methods are essential for exploring complex systems and predicting their reactions.
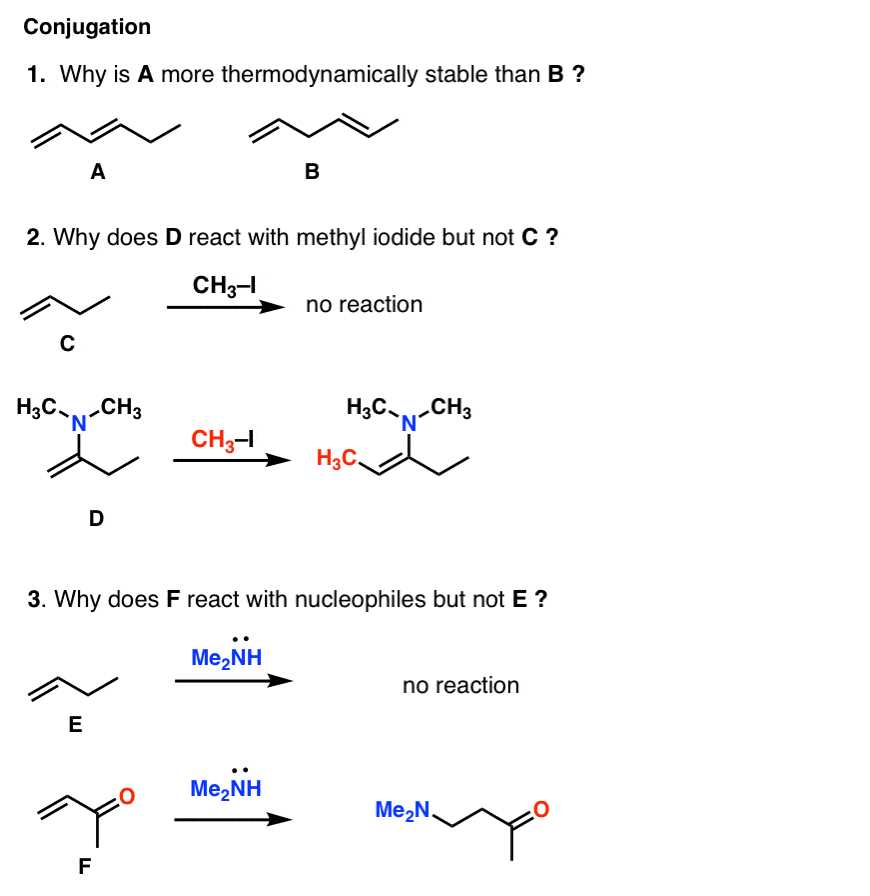
Understanding Functional Group Transformations
The ability to manipulate functional groups and transform them into different forms is crucial in understanding complex molecular reactions. These transformations allow for the synthesis of new compounds, facilitating the development of various materials and pharmaceuticals. Mastery of functional group changes not only enhances your understanding of molecular behavior but also improves your ability to predict reaction outcomes.
Common Functional Group Changes
Below is a table highlighting some common transformations involving functional groups and their general reactivity:
Transformation Functional Group Involved Example Reaction Oxidation Alcohol to Aldehyde/Ketone Alcohol + O₂ → Aldehyde Reduction Aldehyde/Ketone to Alcohol Aldehyde + H₂ → Alcohol Substitution Halide to Alcohol R-Cl + H₂O → R-OH Hydration Alkene to Alcohol Alkene + H₂O → Alcohol Key Principles in Transformations

When considering functional group transformations, it’s essential to understand the driving forces behind these reactions. Some key principles include:
- Electron transfer: The movement of electrons plays a central role in changing bonds and creating new structures.
- Reactivity of functional groups: Some functional groups are more reactive than others, influencing the direction and speed of transformations.
- Environmental conditions: Factors like temperature, solvent, and catalysts can significantly affect the outcome of a transformation.
By becoming familiar with these transformations and their underlying principles, you will gain a deeper understanding of how molecular structures evolve and interact in various chemical processes.
Tips for Solving Stereochemistry Problems

Stereochemistry is a fundamental aspect of molecular structure that focuses on how atoms are arranged in three-dimensional space. It involves understanding how these spatial arrangements impact the properties and reactivity of compounds. To solve stereochemistry problems efficiently, it’s essential to develop a systematic approach that allows you to analyze the spatial configuration of molecules and predict their behavior in reactions.
Here are some valuable tips to help you tackle stereochemistry problems:
- Know the basic terminology: Familiarize yourself with terms such as chiral, achiral, enantiomers, diastereomers, and stereocenters. These are essential for identifying and differentiating molecules.
- Practice drawing structures: Draw molecules in three dimensions, using tools like wedges and dashes to represent bonds that go above or below the plane of the paper. This helps visualize the 3D nature of the molecule.
- Use the Cahn-Ingold-Prelog system: Apply the R/S system to assign the configuration of chiral centers. This systematic approach helps avoid confusion when determining the stereochemistry of a molecule.
- Focus on symmetry: Identify symmetry in molecules to determine if they are chiral or achiral. Molecules with a plane of symmetry are typically achiral, simplifying the problem.
- Understand optical activity: Know how the orientation of chiral centers affects the way molecules interact with polarized light. This can help you identify enantiomers or distinguish between different stereoisomers.
By following these tips and regularly practicing problems, you can develop a strong foundation in stereochemistry and improve your ability to solve related questions with confidence.
Approaching Multi-Step Synthesis Questions
Multi-step synthesis problems require a strategic approach, as they involve creating complex molecules from simpler starting materials through a series of chemical reactions. The key to solving these problems is understanding the transformation of functional groups, the order in which reactions should occur, and how intermediates are formed and transformed throughout the process. These types of questions test both your knowledge of various reactions and your ability to plan and sequence them effectively.
To tackle multi-step synthesis problems, follow these steps:
Planning Your Approach
- Identify the target molecule: Start by carefully examining the final product. Break down its structure to understand which functional groups need to be introduced, modified, or removed.
- Work backwards: One of the most effective strategies is to work backwards from the target molecule. Consider which reactions could have led to this structure and what starting materials could have been used.
- Consider functional group transformations: Focus on the functional groups in both the starting materials and target molecule. Determine how to convert one group to another using specific reagents and reactions.
Choosing the Right Reactions
- Match reactions to objectives: For each step, select the reaction that achieves the desired transformation, such as nucleophilic substitutions, eliminations, or additions.
- Minimize side reactions: Make sure the selected reactions are selective, avoiding unwanted side reactions that may complicate the synthesis.
- Think about reagents and conditions: Consider the reagents and reaction conditions that best facilitate the transformations at each step, ensuring that each reaction step proceeds smoothly.
With consistent practice, this logical and step-by-step approach will improve your ability to solve multi-step synthesis problems and help you excel in these types of questions.
Importance of Practice in Reaction Analysis
Mastering the understanding of chemical transformations requires consistent practice. Through repeated exposure to various reaction mechanisms and their outcomes, you develop the ability to predict and interpret results more effectively. A solid grasp of these processes not only improves your reaction prediction skills but also enhances your problem-solving approach in more complex scenarios. Regular practice allows you to familiarize yourself with patterns, helping you recognize key steps in transformations and avoid common mistakes.
Frequent practice offers several benefits:
- Reinforces foundational knowledge: Regular practice helps solidify the principles behind each reaction, making them easier to recall when faced with new problems.
- Increases reaction speed: The more you practice, the quicker you can analyze reaction sequences, allowing you to solve problems more efficiently during timed assessments.
- Improves troubleshooting skills: With experience, you become better at identifying potential issues in reaction setups and understanding how to correct them.
- Boosts confidence: As you practice more, your ability to approach new and unfamiliar problems increases, building your confidence in solving reaction-related questions.
To optimize your practice, it is important to focus on understanding the underlying mechanisms of each reaction and recognizing the subtle differences between similar processes. This approach ensures that you not only memorize reactions but also understand why and how they occur, which is crucial for success in complex scenarios.
Incorporating different types of problems and scenarios into your practice routine helps you develop a well-rounded understanding of reactions and strengthens your ability to analyze and solve them efficiently.
Efficient Note-Taking for Organic Chemistry
Effective note-taking is an essential skill for mastering complex subjects, especially when dealing with intricate reactions and mechanisms. The goal is to capture key concepts in a clear and organized manner, making it easier to review and retain critical information. By developing a structured approach to note-taking, students can improve their understanding, speed up the learning process, and have valuable resources for future review.
Here are some strategies for taking efficient notes:
- Focus on key concepts: Instead of writing down everything, focus on the most important points, such as reaction mechanisms, reagent types, and functional group transformations. Keep your notes concise but informative.
- Use diagrams and structures: Visuals are powerful tools for understanding complex structures and reactions. Draw reaction arrows, intermediates, and molecular structures to enhance your comprehension.
- Organize by topic: Group related reactions and concepts together. This organization will help you see connections between different topics and make reviewing easier.
- Highlight important reactions: Use color coding or highlighting to emphasize key reactions and their conditions. This will make it easier to locate them when studying.
In addition to the above strategies, regular review and active engagement with your notes are key to ensuring long-term retention. Instead of passively rereading, challenge yourself by summarizing the material, explaining it to others, or applying it to practice problems.
By using these methods, you’ll create a set of notes that not only capture critical information but also help you develop a deeper understanding of the subject, making future study sessions more effective and efficient.