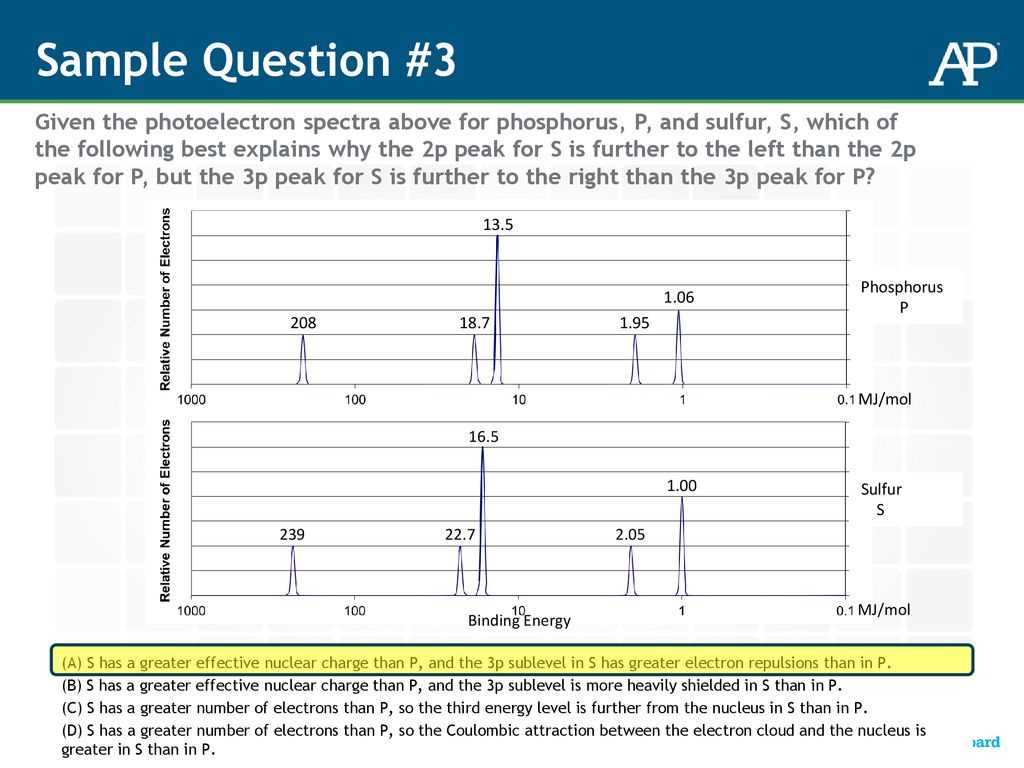
In advanced science courses, understanding the behavior of electrons and their interaction with different energy forms is crucial for mastering key concepts. This section delves into the study of how electrons respond when exposed to external energy, providing insight into the structure of atoms and molecules. Through interactive learning methods, students can develop a deeper grasp of these phenomena, making complex topics more accessible.
One of the most effective approaches to studying these interactions involves active participation in problem-solving exercises. By applying theoretical knowledge to practical scenarios, learners can better understand the principles behind electron emission and energy transitions. This method also helps to enhance critical thinking skills, allowing students to connect theory with real-world applications.
As students explore these concepts, they will uncover the connections between energy absorption, electron removal, and the resulting effects on atomic structure. By gaining a solid foundation in these areas, learners can build the necessary skills to tackle more advanced topics in the field of atomic and molecular physics.
Photoelectron Spectroscopy in AP Chemistry
In advanced science courses, understanding how atoms and molecules interact with light is fundamental to grasping the underlying principles of atomic structure and bonding. By studying how electrons are ejected from an atom when exposed to specific energy sources, students can gain valuable insight into the arrangement of electrons within an atom. This knowledge not only enhances their comprehension of atomic behavior but also serves as a foundation for exploring more complex topics in molecular physics.
The process of measuring the energy of ejected electrons reveals critical information about an atom’s electronic structure, including the energy levels and how electrons are distributed. This approach allows students to predict the behavior of different elements and compounds when subjected to energy variations. In an academic context, mastering this technique helps build a strong understanding of atomic theory and its practical applications in real-world scenarios.
| Element | Binding Energy (eV) | Electron Configuration |
|---|---|---|
| Hydrogen | 13.6 | 1s1 |
| Oxygen | 13.6 | 1s2 2s2 2p4 |
| Carbon | 11.3 | 1s2 2s2 2p2 |
By analyzing data from experiments, students can evaluate trends in electron binding energies across different elements, allowing them to understand periodic trends and how they relate to an atom’s ability to hold onto electrons. This information is not only valuable for academic purposes but also plays a crucial role in the development of technologies such as material science and energy storage systems.
Understanding Key Concepts in Photoelectron Spectroscopy
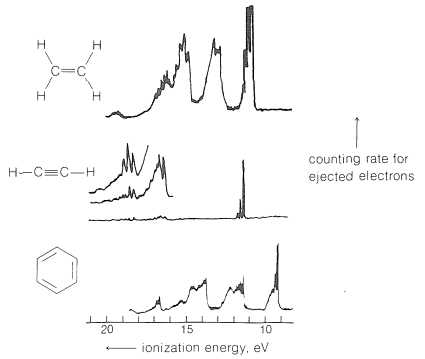
In the study of atomic structure, one of the most important techniques involves analyzing how energy affects the behavior of electrons. This process allows us to explore the arrangement of electrons in atoms and molecules. By understanding how electrons respond to external energy, students can gain valuable insights into the deeper workings of atomic interactions and energy transitions. This knowledge forms the basis for a wide range of scientific applications, from basic research to industrial innovations.
The Role of Energy Levels in Electron Emission
The energy required to remove an electron from its atomic orbit is crucial in understanding electron behavior. These energy levels, often referred to as binding energies, depend on the element in question and the position of the electron within the atom. Elements with different atomic structures exhibit distinct energy patterns when electrons are ejected, making it possible to identify and differentiate between substances based on their electronic configurations.
Interpreting Spectral Data for Atomic Structure
Once the electrons are ejected, the resulting data provides valuable information about the atom’s structure. By examining the energy of emitted electrons, students can deduce the number of electrons in various orbitals and their relative energy levels. This process involves careful analysis of peaks and shifts in the spectral data, allowing for accurate identification of atomic and molecular components. Understanding how to interpret these spectra is key to mastering the principles behind electron dynamics in a variety of substances.
How POGIL Enhances Chemistry Learning
Active learning techniques have proven to be highly effective in helping students understand complex scientific concepts. One such approach focuses on collaboration and problem-solving, where students engage with the material in a dynamic, hands-on manner. This method encourages students to work together, analyze data, and discuss key principles, leading to a deeper understanding of the subject matter. By shifting from passive reception of information to active participation, students not only retain knowledge more effectively but also develop critical thinking and analytical skills.
In this type of learning environment, students take on more responsibility for their education, moving beyond traditional lecture-based instruction. They are encouraged to explore ideas, ask questions, and provide explanations, which promotes a more comprehensive grasp of the material. Working in teams helps to reinforce concepts through peer-to-peer interaction, making complex ideas more accessible and encouraging independent thought.
Moreover, this approach integrates real-world applications, showing students how theoretical knowledge is used in practical situations. By solving problems related to real chemical processes and phenomena, students gain a clearer perspective on how these concepts apply outside the classroom, further enriching their learning experience.
Exploring the Photoelectric Effect in Depth
The photoelectric effect refers to the phenomenon where light, when shined upon certain materials, causes the emission of electrons. This fundamental concept plays a crucial role in understanding how matter interacts with electromagnetic radiation. By studying this effect, we gain insight into the relationship between energy and matter at the atomic level. The process reveals how different wavelengths of light can impart enough energy to free electrons from the material’s surface, providing a clear example of how energy transitions can alter the state of a substance.
At the core of this effect lies the principle that the energy of incoming photons must exceed a certain threshold to release an electron. This threshold varies depending on the material, and understanding it requires careful analysis of the energy levels and properties of the material being studied. The observation of the photoelectric effect led to groundbreaking discoveries in quantum mechanics, helping to shape modern theories of light and matter interaction.
By experimenting with various materials and light sources, students can observe how different factors such as light intensity and frequency affect the rate of electron emission. This hands-on approach allows for a deeper understanding of the underlying principles that govern this phenomenon and its application in technologies such as solar cells, electron microscopes, and more.
The Role of Energy Levels in Chemistry
Energy levels are a fundamental concept in the study of matter, playing a key role in determining how atoms and molecules interact. These levels refer to the specific energies that electrons in an atom or molecule can possess. Understanding the distribution and behavior of electrons in different energy states is essential for explaining chemical reactions, bonding, and the physical properties of substances. The energy required to move an electron from one level to another helps to determine the stability and reactivity of different elements and compounds.
Electron Behavior and Energy Transitions
When an atom or molecule absorbs energy, electrons can move from lower to higher energy levels. This transition is crucial in understanding many physical phenomena, such as the emission of light and the formation of bonds. Some key points about electron transitions include:
- The amount of energy required to move an electron is specific to each element and is related to the spacing between energy levels.
- Electrons can only exist in discrete energy states; they cannot occupy intermediate values.
- The energy difference between levels often corresponds to the energy of light absorbed or emitted in various reactions.
Impact on Chemical Properties and Reactions
The arrangement of electrons in these energy levels determines how atoms interact with each other. For example, atoms with similar energy configurations are more likely to form bonds, while those with significantly different energy levels may struggle to combine. In chemical reactions, the energy released or absorbed during bond formation or breaking is directly linked to these transitions. Understanding these concepts allows scientists to predict how substances will behave in different conditions, such as when exposed to heat, light, or electric fields.
Overall, mastering the principles behind energy levels and electron transitions is crucial for understanding not only atomic structure but also the nature of chemical processes that govern the physical world around us.
Using POGIL for Active Learning in AP Chemistry
Active learning techniques are designed to engage students in the learning process, encouraging them to take a more hands-on approach to studying complex subjects. One effective method involves group-based activities that allow students to collaboratively explore and solve problems. This approach not only promotes deeper understanding but also helps students develop critical thinking, communication, and problem-solving skills. By encouraging interaction with the material and with each other, students can grasp challenging concepts more easily and apply their knowledge in practical scenarios.
Through structured group work, students are able to engage with specific tasks that require them to analyze data, discuss their findings, and come to collective conclusions. This type of learning provides a richer, more interactive experience compared to traditional lecture-based methods. By working together, students can clarify doubts, reinforce their understanding, and gain new insights from their peers. The collaborative nature of this learning strategy also fosters a sense of community and shared responsibility for the learning process.
Benefits of Active Learning Methods:
- Improved retention: Students are more likely to retain information when they actively engage with the material.
- Enhanced problem-solving skills: Working through real-world scenarios helps students refine their ability to analyze and solve problems.
- Better understanding of concepts: Active participation helps break down complex topics into manageable parts, making them easier to understand.
- Development of teamwork skills: Collaborative learning builds communication and teamwork abilities, which are essential in both academic and professional settings.
By utilizing these interactive strategies, students not only enhance their grasp of scientific concepts but also develop the skills needed to succeed in both their academic careers and future endeavors.
Identifying Electron Emission in Spectroscopy
When atoms or molecules interact with energy, such as light or electricity, electrons may be ejected from their original positions. This phenomenon is key to understanding the relationship between energy and the atomic structure of elements. By studying the emission of electrons, scientists can gain valuable insights into the energy levels and configurations within atoms. Identifying these emitted electrons allows researchers to learn more about the material’s properties, including how atoms are arranged and how they interact with external forces.
How Electron Emission Occurs
The process begins when an atom absorbs energy, causing electrons to move to higher energy levels. If the energy is sufficient, it can eject the electron entirely from the atom. The energy required to remove an electron depends on the material and its electronic structure. Understanding the details of electron emission is important for identifying the underlying atomic structure. Key factors include:
- Threshold Energy: Each material has a specific energy threshold that must be exceeded for an electron to be emitted.
- Energy of Emitted Electrons: The energy of the emitted electron provides insight into the electron’s original state within the atom.
- Material Properties: The composition and structure of the material influence how easily electrons are emitted.
Applications in Identifying Elements and Compounds
By measuring the energy of the emitted electrons, scientists can determine specific characteristics of the atoms involved. This data helps to identify which elements are present in a sample, as each element has a unique set of energy levels and electron configurations. Additionally, comparing the emission patterns from different materials can reveal how different substances behave under various energy conditions, offering a deeper understanding of their chemical and physical properties.
In many cases, this method is employed in various fields such as material science, energy research, and environmental studies, providing essential data about the atomic makeup of substances. This technique remains a vital tool in advancing our understanding of atomic theory and its applications in modern technology.
The Significance of Binding Energy Calculations
Binding energy plays a crucial role in understanding the stability and behavior of electrons within atoms and molecules. It refers to the amount of energy required to remove an electron from its bound state to an unbound or free state. By calculating the binding energy, researchers can gain valuable insights into the forces holding electrons in place, as well as the overall structure and properties of a material. This concept is essential for interpreting various physical and chemical phenomena, from atomic interactions to reactions involving the release of energy.
Understanding the Binding Energy of Electrons
The binding energy of an electron is determined by the strength of the interaction between the electron and the nucleus or the surrounding electron cloud. The higher the binding energy, the more energy is required to free the electron. Binding energy can vary depending on several factors, including:
- Atomic Structure: Electrons closer to the nucleus generally experience stronger attraction and thus have higher binding energy.
- Electron Shielding: Electrons in inner orbitals may shield outer electrons, reducing the binding energy for outer electrons.
- Element Type: Different elements have distinct binding energies due to variations in nuclear charge and electron configuration.
Applications in Material Identification and Analysis
Binding energy calculations are essential tools in identifying elements and compounds. By measuring the energy required to remove an electron, scientists can determine the electron configuration and, in some cases, the chemical identity of a substance. These measurements are particularly useful in areas like material science, where understanding the binding energy can help in designing more efficient materials and energy systems.
Additionally, binding energy calculations are applied in fields such as surface science and nanotechnology, where knowing how atoms and molecules interact at the surface level is key. This understanding helps to inform the development of new technologies, from energy storage devices to advanced sensors.
Analyzing Spectral Data from Photoelectron Experiments
In experiments where energy is used to remove electrons from atoms or molecules, the resulting data can provide crucial insights into the electronic structure of a substance. Spectral data collected from these experiments reveals the energies of emitted particles, helping scientists to understand how electrons are arranged and how they interact with their environment. Analyzing this data involves identifying key features in the spectra, which can be correlated to specific energy levels and chemical properties of the material being studied.
Understanding the Key Features of Spectral Data
The spectral data obtained in these experiments typically consist of peaks that correspond to different energy states of electrons. By interpreting the location and intensity of these peaks, researchers can infer important details about the atomic structure and the binding energies of the electrons. Some key aspects to consider when analyzing spectral data include:
- Peak Position: The position of each peak on the energy scale indicates the binding energy of the electron that was emitted.
- Peak Intensity: The intensity of a peak provides information about the number of electrons with a given energy.
- Multiplicity of Peaks: Multiple peaks may indicate the presence of different electron states or interactions within the material.
Using Data for Material Identification and Analysis
Once the spectral data is collected and analyzed, it can be used to identify the material being studied or to gain insight into its chemical composition. The data helps scientists understand how atoms and molecules are structured and how they interact with external energy sources. For example, by comparing the spectra of different substances, it is possible to identify elements based on their unique energy signatures.
Additionally, the spectral data can reveal details about the nature of chemical bonding and the presence of impurities or defects in a material. This information is valuable for a wide range of applications, from developing new materials to improving the efficiency of energy systems.
Sample Data Table: Peak Positions and Intensities
| Peak Position (eV) | Peak Intensity (arbitrary units) | Electron Configuration |
|---|---|---|
| 12.5 | 250 | Core level electron |
| 15.0 | 500 | Outer electron transition |
| 17.8 | 300 | Ionization peak |
By carefully studying the spectral data in this way, scientists can extract detailed information about the composition, bonding, and properties of a material, contributing to the advancement of research in material science and other fields.
Interpreting Peak Shifts in Spectroscopy Graphs
In many experiments where electrons are excited or ejected from atoms or molecules, shifts in the resulting peaks on a graph can provide valuable insights into the nature of the sample being studied. These shifts, whether to higher or lower energy values, are not random but rather indicative of changes in the electronic environment or external factors affecting the sample. By understanding and interpreting these shifts, researchers can gain a deeper understanding of the material’s composition, molecular structure, and interactions with external forces.
Types of Peak Shifts
Peak shifts in the graphs can occur due to various factors, each of which conveys different information about the sample. Some of the most common types of shifts include:
- Redshift: When peaks shift to lower energy, it typically indicates a reduction in the binding energy of the electrons. This can occur when the electron density around the atom decreases, often due to changes in the chemical environment.
- Blueshift: A shift to higher energy values suggests that the electrons are more tightly bound. This can be the result of increased nuclear charge or changes in the bonding environment.
- Resolution Shifts: A shift in the resolution of the peaks may suggest variations in the material’s composition, where different components or impurities are influencing the overall spectral response.
Factors Influencing Peak Shifts
Several factors can cause these shifts, and understanding them is crucial for correct interpretation. These factors include:
- Electron Shielding: The shielding effect of inner electrons can impact the energy levels of outer electrons, leading to shifts in the peaks observed in the spectrum.
- Chemical Bonding: Changes in the chemical environment, such as the formation of new bonds or changes in oxidation states, can cause shifts in the energy levels of electrons.
- External Fields: The application of external electric or magnetic fields can also result in shifts, which may reveal information about the material’s interaction with these forces.
Practical Applications of Peak Shift Analysis
Understanding and interpreting peak shifts is essential for a wide range of applications, from material characterization to chemical analysis. Researchers use peak shifts to identify the nature of chemical bonds, detect impurities, and study the effect of different conditions on a sample. For example, in surface science, peak shifts can help identify how surface atoms behave differently from those in the bulk material, leading to a better understanding of material properties at the microscopic level.
Moreover, the analysis of peak shifts can provide important insights in fields such as nanotechnology, where even small changes in a material’s structure can drastically affect its properties. By interpreting these shifts, scientists can refine their understanding of how materials behave under various conditions and apply this knowledge to develop better, more efficient materials for advanced applications.
Understanding Core and Valence Electrons
In atoms, electrons are distributed across different energy levels or shells. These electrons play a crucial role in determining the chemical behavior of elements. The electrons that are closest to the nucleus are called core electrons, while those in the outermost shell are referred to as valence electrons. Understanding the differences between these two types of electrons is essential for grasping how atoms interact, form bonds, and react with other substances.
The Role of Core Electrons
Core electrons are located in the inner energy levels of an atom, closer to the nucleus. These electrons are tightly bound to the atom due to the strong attractive forces of the positively charged nucleus. As a result, core electrons are not typically involved in chemical reactions, as they do not interact directly with other atoms. However, their presence affects the overall charge distribution and helps maintain the stability of the atom.
- Stability: Core electrons contribute to the overall stability of the atom by shielding the valence electrons from the full effect of the nuclear charge.
- Energy Levels: Core electrons reside in the lower energy levels, which are closer to the nucleus and more tightly bound.
The Importance of Valence Electrons
Valence electrons, on the other hand, are the outermost electrons in an atom and are directly involved in chemical bonding. These electrons determine how an atom interacts with others to form molecules. The number of valence electrons in an atom influences its reactivity and its ability to form bonds with other atoms. For example, atoms with a full valence shell are typically stable and less likely to react, while atoms with few valence electrons are more likely to bond with other atoms to achieve stability.
- Chemical Bonds: Valence electrons are responsible for forming covalent, ionic, and metallic bonds.
- Reactivity: The number of valence electrons determines how readily an atom will participate in chemical reactions.
By understanding the distribution of core and valence electrons, scientists can predict the behavior of elements in chemical reactions and understand their role in forming various substances. This knowledge is essential for fields such as material science, molecular biology, and the development of new technologies.
What Is Ionization Energy and Why It Matters
Ionization energy is the energy required to remove an electron from an atom or molecule in its gas phase. This process is crucial in understanding how atoms interact, bond, and react in various environments. The value of ionization energy reveals much about an element’s reactivity and its tendency to form or lose bonds. By studying this property, we can gain insights into how elements behave chemically and predict how they will respond in different reactions.
Factors Influencing Ionization Energy
Several factors affect the ionization energy of an element. These factors determine how easily an electron can be removed from an atom and provide a deeper understanding of its position within the periodic table.
- Atomic Size: As the size of an atom increases, the outermost electrons are farther from the nucleus and are less tightly bound. This generally leads to a lower ionization energy.
- Nuclear Charge: The higher the positive charge of the nucleus, the more strongly it attracts electrons. Atoms with higher nuclear charge tend to have higher ionization energies.
- Electron Shielding: Electrons in inner shells can shield outer electrons from the full attraction of the nucleus, making it easier to remove an electron from outer shells.
The Importance of Ionization Energy

Ionization energy plays a critical role in many chemical processes, particularly in determining how atoms bond with one another. The ability of an atom to lose or gain electrons affects its reactivity, as well as its stability in different chemical environments.
- Reactivity Trends: Elements with low ionization energies are typically more reactive, as they can easily lose electrons to form positive ions. This is common among metals.
- Electron Configuration: Ionization energy helps explain the arrangement of electrons in atoms and how atoms achieve stable electron configurations.
- Bond Formation: Ionization energy influences an element’s ability to form ionic or covalent bonds, directly impacting molecular structure and properties.
Understanding ionization energy allows scientists to predict the behavior of elements in chemical reactions, design new materials, and even advance technology in areas like battery development and energy storage.
POGIL Techniques for Chemistry Problem-Solving
Effective problem-solving in the study of scientific concepts requires more than memorizing formulas or procedures. A deeper understanding is built by engaging with the material, analyzing it critically, and working collaboratively. This approach allows students to connect theory to practice and develop problem-solving skills that are essential in scientific fields. By using interactive methods that involve active participation, learners can improve their ability to solve complex problems in various scientific disciplines.
One key strategy for enhancing problem-solving skills is the use of structured group activities that encourage collaborative learning. In such activities, students work together to explore concepts, analyze data, and solve problems in real time. This process not only helps students retain information but also strengthens their critical thinking abilities. The role of the facilitator is crucial in guiding the group through the process, ensuring that each member participates and contributes to the collective solution.
Steps Involved in Effective Problem-Solving
When approaching a problem, a systematic approach ensures that all aspects of the issue are addressed comprehensively. The following steps are commonly used in effective problem-solving techniques:
- Understanding the Problem: Before attempting to solve a problem, it is essential to thoroughly understand the problem’s context and what is being asked.
- Breaking Down the Problem: Decomposing the problem into smaller, more manageable parts helps in identifying key concepts and relationships.
- Collaborative Discussion: Working with peers fosters different perspectives, allowing for a more well-rounded approach to finding solutions.
- Testing Solutions: After generating possible solutions, it is crucial to test them and evaluate their effectiveness in addressing the problem.
The Benefits of Collaborative Problem-Solving
Working in groups not only enhances understanding but also cultivates teamwork, communication, and the ability to think critically under pressure. Some of the key benefits include:
- Active Engagement: Collaborative problem-solving promotes deeper engagement with the material, encouraging students to think critically and independently.
- Increased Retention: Active learning has been shown to increase retention rates, as students are more likely to remember information they’ve actively discussed and applied.
- Real-World Application: Working through real-world problems helps students relate theoretical knowledge to practical scenarios they may encounter in future studies or careers.
By integrating these techniques into their study habits, students can significantly improve their problem-solving skills, becoming more confident and capable in applying their knowledge in diverse scientific contexts.
Comparing Atomic and Molecular Spectra
The interaction of light with matter results in the emission or absorption of energy, which is often observed in the form of spectra. These spectra can be used to study the internal structure of materials, revealing information about their composition and properties. However, the way light interacts with individual atoms differs significantly from how it interacts with molecules. This leads to distinct spectral patterns, each offering unique insights into the nature of the substance being studied. By comparing the spectra of atoms and molecules, one can better understand the differences in their energy levels and the transitions that occur when light is absorbed or emitted.
Key Differences Between Atomic and Molecular Spectra
When examining the spectra produced by atoms and molecules, there are several important differences to consider. These differences arise due to the nature of the energy transitions involved and the level of complexity within the substances themselves. Below are the key aspects that distinguish atomic and molecular spectra:
| Characteristic | Atomic Spectra | Molecular Spectra |
|---|---|---|
| Energy Transitions | Electron transitions between discrete energy levels | Transitions involving electrons, vibrations, and rotations |
| Appearance | Sharp, well-defined spectral lines | Broader, more complex bands with multiple peaks |
| Temperature Sensitivity | Requires higher temperatures for emission spectra | Absorption spectra can be measured at lower temperatures |
| Complexity | Relatively simple with fewer transitions | More complex, incorporating rotational and vibrational states |
Applications and Significance
Both atomic and molecular spectra have significant practical applications in various fields of science. Atomic spectra are crucial for identifying elements in unknown substances, as each element produces a unique set of spectral lines. Molecular spectra, on the other hand, provide insight into the structure and behavior of molecules, including their bonding and the nature of their interactions in different states of matter. These spectra are instrumental in understanding chemical reactions, material properties, and the behavior of gases and liquids under various conditions.
By analyzing these spectra, scientists can uncover vital information about the composition, structure, and behavior of materials, which is essential in fields ranging from material science to environmental studies and even astrophysics.
Common Mistakes in Spectroscopy Interpretations
Interpreting data from experiments that involve light interaction with matter can be challenging. While these experiments provide valuable insights into the structure and properties of substances, it is easy to make errors in analysis that can lead to incorrect conclusions. Understanding the common pitfalls in interpreting these results can help improve accuracy and ensure that the data is properly understood. From misidentifying peaks to overlooking key factors, several common mistakes can occur when analyzing spectra.
1. Misidentifying Peak Positions
One of the most frequent errors in spectrum interpretation is the misidentification of peak positions. Spectral lines correspond to specific transitions within a substance, and each line represents a distinct energy change. Misreading the position of a peak can lead to confusion about the energy levels involved and can result in incorrect conclusions about the substance’s composition or behavior. It’s essential to accurately calibrate instruments and carefully analyze the data to avoid this mistake.
2. Overlooking Instrumental and Environmental Factors
Instrumental settings, such as resolution or sensitivity, can significantly affect the appearance of a spectrum. A common mistake is to assume that a spectrum is purely the result of the sample being analyzed, without considering how the equipment might influence the data. Environmental factors, such as temperature, pressure, and even sample preparation, can also alter the spectrum. Ignoring these factors can lead to inaccurate interpretations. Always account for the experimental conditions when analyzing results to ensure reliable conclusions.
3. Ignoring Overlap of Peaks
Another common mistake is neglecting the overlap of peaks from different sources or transitions. In complex spectra, several different transitions may occur at nearly the same energy level, leading to peaks that overlap. Without properly distinguishing between these overlapping features, it can be difficult to pinpoint the exact cause of a peak and assign it to the correct transition. Careful analysis and, when necessary, additional techniques like computational simulations or higher resolution measurements are essential to avoid confusion.
4. Misinterpreting Intensities
The intensity of a peak is often linked to the abundance or concentration of the species involved. However, factors such as instrumental calibration and sample thickness can affect intensity readings. Assuming that peak intensity directly corresponds to the quantity of a substance without considering these factors may lead to errors in determining concentrations or concentrations of various compounds. Understanding how external factors influence intensity is crucial for accurate quantitative analysis.
5. Failing to Account for Matrix Effects
The surrounding matrix of a sample can have a significant impact on the results observed. In some cases, the presence of other substances or compounds in the sample may distort the spectrum, creating additional peaks or altering the position of existing ones. Ignoring these matrix effects can result in false conclusions. It is important to perform a careful sample preparation process and, when applicable, use reference materials or standards to correct for these influences.
By being aware of these common errors and taking steps to avoid them, researchers and students can ensure that their analysis of experimental data is accurate and reliable, leading to more robust and meaningful interpretations of spectra.
Practical Applications of Electron Emission Analysis
The study of electron interactions with matter offers profound insights into the composition and behavior of various materials. This technique plays a crucial role in a wide range of scientific and industrial fields, providing valuable data on atomic and molecular structures, electronic configurations, and material properties. By analyzing the emission of electrons from a sample, it becomes possible to determine characteristics such as elemental composition, bonding types, and chemical environments in a non-destructive manner. These applications extend from material science to electronics, and even surface analysis in nanotechnology.
1. Material Surface Characterization
One of the most significant uses of this analytical method is in the detailed examination of material surfaces. It is particularly useful in studying thin films, coatings, and layered materials. Through electron emission analysis, scientists can determine the elemental composition of the outermost layers of a material, identify contamination or impurities, and examine the chemical states of the elements present. This is invaluable for industries that rely on surface properties, such as semiconductor manufacturing, corrosion testing, and surface coating technologies.
2. Semiconductor Industry and Electronics
The semiconductor industry benefits greatly from this type of analysis, as it helps in understanding the electronic properties of materials used in devices such as microchips and transistors. By evaluating the energy levels and binding energies of electrons in semiconductor materials, engineers can fine-tune the manufacturing processes for improved performance and reliability. In particular, this technique provides insight into doping levels, interface quality, and defect structures in semiconductor devices, which are crucial for the development of more efficient and smaller electronic components.
3. Environmental and Analytical Chemistry
In environmental analysis, electron emission techniques are used to identify pollutants and trace elements in various environmental samples. This method enables scientists to analyze contaminants at very low concentrations, providing essential data for monitoring air quality, water purity, and soil contamination. It also plays a role in analyzing the chemical composition of complex samples, such as those found in pharmaceuticals, food products, and natural resources.
4. Nanotechnology and Surface Science
In the realm of nanotechnology, understanding surface properties at the atomic level is essential for developing new materials and devices. Electron emission analysis aids in investigating the electronic structures of nanomaterials, such as carbon nanotubes, graphene, and quantum dots. This knowledge is crucial for the design of advanced nanodevices, including sensors, catalysts, and energy storage devices, where surface reactivity and electronic behavior are of paramount importance.
In conclusion, electron emission analysis serves as an indispensable tool in various scientific fields, enabling precise material characterization, enhancing the performance of electronic devices, and contributing to innovations in nanotechnology and environmental monitoring. Its broad range of applications demonstrates its vital role in advancing technology and scientific research.