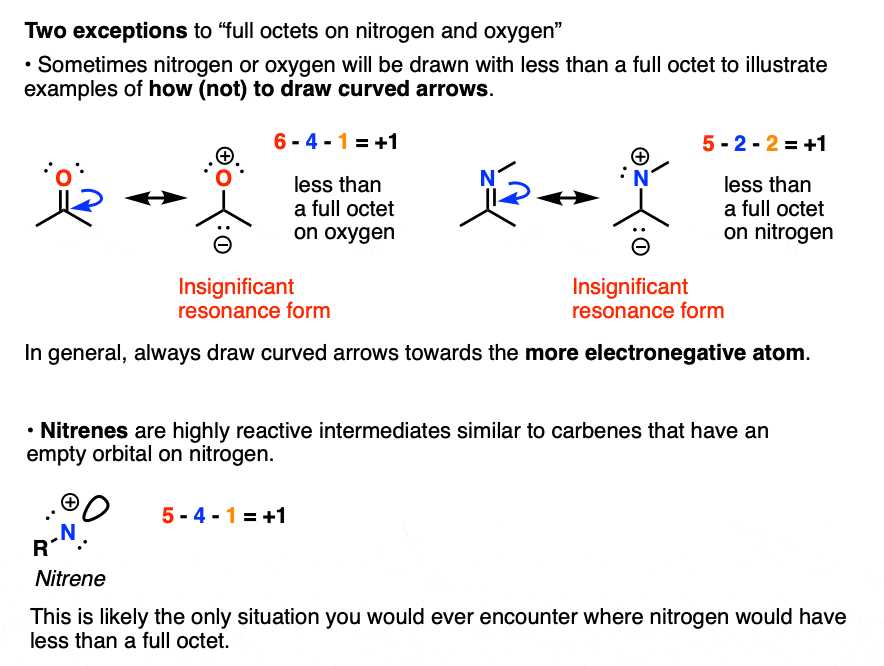
In this section, we explore the fundamental principles that govern the structure and organization of matter at the microscopic level. The arrangement of particles within an element plays a critical role in determining its properties and behavior. A closer look at these principles reveals the intricate relationships between energy, matter, and the underlying forces at play.
By delving into the specifics of how particles interact and occupy specific regions, we gain insight into the underlying rules that define chemical bonding and reactivity. These interactions are crucial in explaining the characteristics of various substances and their behavior in different environments. Understanding these concepts lays the groundwork for more advanced topics in chemistry and physics.
Chapter 5 Electrons in Atoms Answer Key
This section focuses on the fundamental concepts that govern the arrangement and behavior of subatomic particles within elements. These principles are essential for understanding how particles are distributed and how they influence the properties of matter. By examining the rules and patterns of these interactions, we can gain a deeper appreciation for the structure of matter and its behavior in various contexts.
In this context, we look at the way particles are organized into different energy levels and orbitals. These arrangements follow specific rules that help explain the chemical and physical characteristics of substances. Understanding these patterns allows for a clearer picture of how substances react and interact with each other in different environments, laying the foundation for more advanced studies in chemistry and physics.
Overview of Atomic Structure
The fundamental building blocks of matter are arranged in a highly organized and structured manner. This arrangement determines how substances interact and behave. At the heart of these interactions lies the organization of small particles that make up each element, each following specific patterns and rules that govern their properties.
Understanding the basic structure involves looking at how these particles are distributed across different regions and energy levels. The configuration of these regions defines the characteristics and reactivity of the material. Here are some key concepts to consider:
- Core Structure: The central part of any element contains positively charged particles, surrounded by negatively charged regions.
- Energy Levels: These regions, or shells, hold particles at varying distances from the core, each corresponding to different energy states.
- Subatomic Arrangement: The way these particles are arranged in layers or orbitals defines the overall stability and behavior of a substance.
In essence, the way particles are arranged within an element helps explain its properties, how it reacts with other substances, and its role in the larger picture of chemical processes. This foundational knowledge is essential for understanding both simple and complex reactions in the natural world.
Understanding Electron Energy Levels

The arrangement of subatomic particles within an element is governed by specific energy states, which dictate how these particles behave and interact. These energy states are not random but instead follow well-established patterns, shaping the properties of matter. Understanding how energy is distributed among the particles is crucial to grasping the behavior of substances and their reactions.
The Concept of Energy States
Each particle within an element occupies a distinct energy level, with particles in higher levels having more energy compared to those in lower levels. These levels are often visualized as concentric shells around the nucleus, where each shell can hold a specific number of particles. The further a shell is from the center, the higher the energy associated with it. This arrangement helps determine how the substance reacts with other elements, especially in chemical bonding.
Energy Transitions and Stability
Particles can move between these energy levels by absorbing or releasing energy. When particles gain enough energy, they jump to a higher level; conversely, they release energy when they fall to a lower level. These transitions are fundamental to understanding many phenomena, including light emission and absorption. The stability of a material also depends on how its particles are distributed across these energy levels, which ultimately influences its chemical and physical properties.
Atomic Orbitals and Their Shapes
The arrangement of subatomic particles in an element is not only determined by their energy levels but also by specific regions where they are most likely to be found. These regions, known as orbitals, have distinct shapes that influence the behavior and interaction of particles. The shape of each orbital is key to understanding how these particles form bonds and participate in reactions.
Types of Orbitals
There are several types of orbitals, each with its own unique shape. The most common orbital types are s, p, d, and f orbitals. Each of these orbitals can hold a specific number of particles, and their shape determines how they interact with other orbitals and particles. Below is a basic overview of the most important orbital types and their respective shapes:
| Orbital Type | Shape | Number of Particles |
|---|---|---|
| s | Sphere | 2 |
| p | Figure-eight | 6 |
| d | Clovershaped | 10 |
| f | Complex, multiple lobes | 14 |
Significance of Orbital Shapes
The shape of each orbital directly impacts how particles interact with each other. For example, orbitals with different shapes can overlap to form chemical bonds, which are crucial in determining the properties of substances. The spatial arrangement of these orbitals also explains why some elements behave differently in chemical reactions. Understanding orbital shapes is thus essential for a deeper comprehension of both chemical and physical processes.
Electron Configuration in Atoms
The arrangement of subatomic particles within an element follows specific patterns that are crucial for understanding its chemical and physical properties. This arrangement, or distribution, within various energy levels and orbitals determines how these particles interact with other substances. By organizing particles according to energy and spatial regions, we gain insight into an element’s behavior in reactions and its stability.
Building the Configuration
The distribution of particles is governed by a set of rules that prioritize energy efficiency and stability. These particles fill available regions starting from the lowest energy level and progressing to higher ones. The configuration can be represented through a series of numbers and letters, indicating the energy levels and orbitals occupied. Below is a general guide for filling orbitals in the correct sequence:
| Orbital Type | Energy Level | Maximum Capacity |
|---|---|---|
| s | 1 | 2 |
| p | 2 | 6 |
| d | 3 | 10 |
| f | 4 | 14 |
Significance of Configuration
The configuration of particles within an element not only defines its stability but also determines its chemical reactivity. Elements with similar configurations in their outer regions often exhibit similar properties, which explains periodic trends observed in the periodic table. By understanding the configuration, scientists can predict how elements will behave in different reactions, making this concept fundamental in both chemistry and physics.
Pauli Exclusion Principle Explained
In the realm of subatomic particles, there are specific rules that govern how they can be arranged within different regions or orbitals. One fundamental rule is the Pauli Exclusion Principle, which helps explain the limitations and behavior of these particles. This principle asserts that no two particles in the same region can occupy the same state simultaneously, ensuring a unique configuration for each particle.
Essentially, the principle states that each particle within an element must have distinct quantum properties, which include its energy level, orbital shape, and orientation. This rule plays a crucial role in determining the structure and stability of matter, as it prevents overcrowding within orbitals and allows for a more orderly arrangement of particles.
Key Implications of the Pauli Exclusion Principle
The application of this principle results in several important effects on matter:
- Orbital Occupation: No orbital can hold more than two particles with opposite spins.
- Spatial Arrangement: Particles must be distributed across available orbitals, with higher energy levels being occupied only when lower ones are filled.
- Atomic Stability: The exclusion principle contributes to the overall stability of elements, ensuring they don’t become overly energetic or unstable.
This principle is fundamental in understanding not only the structure of matter but also the behavior of elements in chemical reactions, as it helps explain the arrangement of particles within orbitals and how these arrangements influence reactivity and bonding.
Hund’s Rule and Its Application
In the study of subatomic particle behavior, certain principles dictate how particles distribute themselves across available energy states. One of these important rules is Hund’s Rule, which governs the way particles fill orbitals within a given energy level. It specifies that when multiple orbitals of the same energy are available, particles will first occupy different orbitals with parallel spins before pairing up in the same orbital.
This rule plays a vital role in ensuring the stability and lowest energy configuration of particles within an element. By following Hund’s Rule, particles are arranged in a way that minimizes repulsion, leading to a more stable and energetically favorable configuration. The principle is particularly relevant when considering how particles behave in complex chemical environments and when forming bonds.
Practical Applications of Hund’s Rule
The application of Hund’s Rule can be observed in various chemical and physical phenomena, including:
- Orbital Filling: Particles tend to occupy available orbitals singly before pairing up, resulting in an overall more stable configuration.
- Magnetic Properties: The rule helps explain the magnetic behavior of substances, as the arrangement of particles can influence whether a material is magnetic or not.
- Chemical Bonding: The distribution of particles in orbitals plays a key role in the formation of chemical bonds, determining how atoms interact to form molecules.
Hund’s Rule helps explain how matter is organized and why certain elements exhibit specific characteristics in chemical reactions. By following this rule, we gain deeper insights into the behavior of particles, the structure of substances, and their reactivity in different environments.
Bohr’s Model of the Atom
The Bohr model introduced a groundbreaking approach to understanding the structure of matter at the subatomic level. This model proposed that the fundamental particles of an element are arranged in specific, quantized energy levels or orbits around a central nucleus. The concept of distinct energy levels revolutionized the understanding of how matter behaves and interacts with energy, providing a clearer picture of atomic structure.
In Bohr’s model, particles occupy fixed orbits with a defined energy. These orbits are not random but are determined by specific rules, with each level corresponding to a particular energy state. When particles absorb or release energy, they move between these orbits, a concept that helped explain phenomena such as spectral lines and energy transitions in various elements.
Key Features of Bohr’s Model
Bohr’s model presented several important concepts that laid the foundation for modern atomic theory:
- Quantized Energy Levels: The model proposed that only specific energy levels are allowed, and particles can only exist in these levels.
- Stable Orbits: Particles within these orbits do not radiate energy, ensuring the stability of the atom.
- Energy Absorption and Emission: When particles absorb or release energy, they jump between different orbits, corresponding to specific wavelengths of light.
While the Bohr model was later refined with more advanced theories, it remains a pivotal step in understanding atomic structure. It introduced the concept of quantized energy levels, which not only explained the stability of matter but also opened the door for further exploration into the behavior of subatomic particles.
Electron Transitions and Emission Spectra
The movement of subatomic particles between different energy levels within an element plays a critical role in determining how it interacts with light and other forms of energy. When these particles absorb or release energy, they undergo transitions, shifting from one energy state to another. These transitions lead to the emission of light, creating a unique spectrum of colors that can be observed and analyzed. The study of these light emissions provides valuable insights into the structure and properties of elements.
Each transition corresponds to a specific energy difference between the levels involved, and the emitted light has a distinct wavelength or frequency. The pattern of these emitted wavelengths forms an emission spectrum, which is unique to each substance. These spectra serve as a fingerprint for identifying elements and understanding their behavior in various physical and chemical processes.
Understanding the Emission Spectrum
The emission spectrum of an element consists of several key features:
- Distinct Lines: Each transition between energy levels results in the emission of light at specific wavelengths, forming a series of lines in the spectrum.
- Energy Differences: The energy emitted or absorbed during a transition is directly related to the difference in energy between the two levels involved.
- Uniqueness of Spectra: No two elements have the same emission spectrum, making it a powerful tool for identification and analysis.
Emission spectra have wide applications in fields such as spectroscopy, astronomy, and chemistry, allowing scientists to analyze the composition of distant stars, identify elements in laboratory samples, and understand fundamental principles of particle behavior.
Quantum Numbers and Their Importance
In understanding the behavior of subatomic particles, quantum numbers play a crucial role in describing their properties and how they interact within various energy states. These numbers provide a set of values that define the specific characteristics of a particle’s position and movement, offering a deeper insight into its energy and spatial distribution. They allow scientists to describe the arrangement of particles in complex systems and predict their behavior in different scenarios.
Quantum numbers are essential for characterizing the different states a particle can occupy within an element, as they indicate both the energy level and the spatial orientation of the state. By understanding these numbers, it becomes possible to explain a variety of phenomena, from chemical bonding to spectral emissions, as well as predict the interactions between different particles.
Types of Quantum Numbers
There are several types of quantum numbers, each serving a distinct purpose in describing the state of a particle:
- Principal Quantum Number (n): Indicates the energy level and distance from the nucleus, with larger numbers corresponding to higher energy levels.
- Angular Momentum Quantum Number (l): Defines the shape of the orbital, determining the angular distribution of the particle’s wave function.
- Magnetic Quantum Number (ml): Describes the orientation of the orbital in space, allowing for the distinction between different orbitals within the same energy level.
- Spin Quantum Number (ms): Represents the intrinsic spin of a particle, which can either be “up” or “down,” defining its magnetic properties.
Each quantum number helps define a unique state for a particle, providing the foundation for understanding atomic structure, chemical behavior, and various physical phenomena. Together, these numbers enable precise calculations and predictions in both theoretical and experimental physics.
Principle of Electron Wave-Particle Duality
The principle of wave-particle duality describes the fundamental concept that particles, such as subatomic entities, can exhibit both wave-like and particle-like behavior depending on the situation. This dual nature challenges traditional classical physics and has profound implications for how we understand the behavior of matter on a microscopic scale. The idea suggests that certain phenomena that occur at the quantum level cannot be fully explained by treating particles solely as objects or waves, but instead require a synthesis of both perspectives.
As a result of this duality, particles can behave like waves under specific conditions, such as diffraction and interference, while also demonstrating characteristics of discrete particles when interacting with other matter. This concept is central to quantum mechanics, where the boundary between waves and particles becomes blurred, fundamentally altering how we view the physical world at its most basic level.
Wave-Like Behavior
When particles exhibit wave-like behavior, they can spread out over space, interfering with each other in patterns typical of light waves. This behavior is most noticeable under certain conditions, such as:
- Diffraction: When particles pass through small openings or around obstacles, they can spread out and create interference patterns, similar to how light behaves.
- Interference: Waves can overlap, reinforcing or canceling each other out, a phenomenon that can also be observed with particles in quantum experiments.
Particle-Like Behavior
Despite their wave-like characteristics, these subatomic entities also exhibit behavior typical of particles, such as:
- Discreteness: When interacting with detectors, particles can appear as discrete, localized events rather than continuous waves.
- Momentum Transfer: In collisions or interactions with other matter, the particle behaves as though it has a defined position and momentum, akin to classical objects.
This duality of behavior is best exemplified in experiments like the famous double-slit experiment, which demonstrates how particles can behave as both waves and particles, depending on the nature of the observation. Understanding wave-particle duality is essential for grasping the core principles of quantum theory and the intricate nature of matter at the most fundamental level.
Energy Levels and Sublevels Breakdown
In quantum mechanics, the concept of energy levels and their subdivisions is essential for understanding how particles are arranged and how they behave in different states. Each energy level represents a specific region around a nucleus where a particle is most likely to be found, and within these levels, there are sublevels that further define the particle’s characteristics, such as its spatial distribution and energy. These structures help explain the behavior of matter, including the formation of chemical bonds and the emission of light.
Energy levels are often represented by quantum numbers, which describe the size and energy of the level, while sublevels further detail the shape and orientation of the orbital paths within those levels. This division allows for a more precise understanding of the possible positions and energy states of particles, essential for explaining both fundamental and complex physical phenomena.
Energy Levels
Energy levels are the broadest divisions in the structure of an atom and are designated by quantum numbers. Each level can hold a limited number of particles, and the energy associated with a level increases as the distance from the nucleus grows. The first level, being closest to the nucleus, holds the least energy, while subsequent levels hold progressively higher energy values. These levels are often represented by the principal quantum number (n).
Sublevels
Within each energy level, there are sublevels, which define the specific shapes and orientations of orbitals that particles can occupy. These sublevels are characterized by different quantum numbers and determine the number of possible orbitals within a level. The main sublevel types include:
- s-sublevel: Spherical in shape, with only one orbital.
- p-sublevel: Dumbbell-shaped, containing three orbitals.
- d-sublevel: Complex in shape, containing five orbitals.
- f-sublevel: Even more complex in shape, containing seven orbitals.
These sublevels increase in complexity as the energy levels progress, providing more possible orbitals for particles to occupy. This structure plays a crucial role in determining how particles interact, leading to the formation of chemical bonds, absorption or emission of light, and other fundamental processes in nature.
Electron Distribution in Multi-Electron Atoms
In more complex structures with multiple particles, the arrangement of these particles within different energy regions becomes significantly more intricate. As the number of particles increases, the ways in which they occupy different energy levels and sublevels must be carefully considered. This distribution not only depends on the available space within the various energy regions but also on the interactions between particles that influence their placement. The study of this distribution provides insight into the physical properties and chemical behavior of various elements.
In multi-particle systems, each particle occupies specific orbitals within sublevels, with the distribution governed by rules that take into account both energy and spatial constraints. As these systems grow in complexity, the particles tend to fill available orbitals according to energy hierarchy, but interactions between them, such as repulsion, also play an essential role in the final configuration.
Principles of Distribution
The distribution of particles in more complex systems follows certain principles. These include the Pauli Exclusion Principle, Hund’s Rule, and the Aufbau Principle. Together, these principles dictate how particles fill orbitals in various energy levels:
- Pauli Exclusion Principle: No two particles can occupy the same quantum state within a given system.
- Hund’s Rule: Particles will occupy orbitals of the same energy level singly before pairing up.
- Aufbau Principle: Particles fill lower energy orbitals first before occupying higher energy orbitals.
Example of Electron Distribution
Consider a multi-particle system such as the configuration of a particular element. The distribution of particles can be represented in terms of the energy levels and sublevels, where each sublevel is filled according to the principles mentioned earlier. Below is a sample table showing the distribution of particles in the energy levels of a system:
| Energy Level | Sublevel | Number of Orbitals | Max. Number of Particles |
|---|---|---|---|
| 1 | s | 1 | 2 |
| 2 | s, p | 1, 3 | 2, 6 |
| 3 | s, p, d | 1, 3, 5 | 2, 6, 10 |
| 4 | s, p, d, f | 1, 3, 5, 7 | 2, 6, 10, 14 |
This table illustrates how the sublevels expand in higher energy levels, providing more space for particles. The distribution in each sublevel helps determine the overall energy configuration of the system and contributes to its chemical and physical properties.
Applications of Electron Configuration
The arrangement of particles within various energy regions plays a crucial role in determining the properties and behavior of elements. Understanding how these particles are distributed allows scientists to predict chemical reactivity, bonding, and even the color of substances. The configuration of a system influences a wide range of phenomena, from the stability of materials to their interaction with light and energy. This understanding is essential not only in chemistry but also in fields like physics and material science.
Electron configuration is particularly important in explaining the periodicity of elements, their ionization energies, and how they bond with other elements to form compounds. The way particles are organized in their respective orbitals determines many physical and chemical properties, including magnetism, conductivity, and the formation of molecular structures.
Chemical Reactivity and Bonding
The distribution of particles within an element’s energy levels directly affects its reactivity. Elements with similar configurations in their outermost energy levels exhibit similar chemical behaviors. For example, elements in the same group of the periodic table tend to form similar compounds due to their shared configuration. Some important applications include:
- Covalent Bonding: Atoms with unfilled orbitals tend to share particles to achieve a more stable configuration, forming covalent bonds.
- Ionic Bonding: When particles are transferred between atoms, forming ions, the configuration of these ions determines the stability of the resulting ionic compound.
- Reactivity Trends: Elements with nearly full or nearly empty outer orbitals are more likely to react in specific ways, such as the tendency of alkali metals to form ionic compounds.
Magnetism and Electrical Conductivity
The arrangement of particles also plays a significant role in determining the magnetic and electrical properties of materials. For example, materials that have unpaired particles in certain orbitals can exhibit magnetic behavior. Additionally, the ease with which particles can move between orbitals influences a material’s ability to conduct electricity. Key applications include:
- Magnetic Materials: Materials like iron are magnetic because of the specific configuration of particles in their orbitals, which allows them to align with external magnetic fields.
- Semiconductors: The ability of certain materials to conduct electricity is controlled by the arrangement of particles in their energy levels. By manipulating electron configuration, materials can be made to act as semiconductors.
- Superconductivity: Some materials exhibit zero electrical resistance at low temperatures due to the special configuration of particles, leading to phenomena like superconductivity.
By understanding the application of electron configuration, scientists can tailor materials and design new compounds for specific purposes, improving everything from electronics to energy production.
Periodic Table and Electron Placement
The arrangement of elements in the periodic table reflects the systematic organization of their fundamental components. This arrangement determines how these components are distributed across different regions, influencing their chemical properties and reactivity. The way these fundamental particles are arranged within various energy levels plays a central role in shaping the behavior of each element. The periodic table provides a framework for understanding how elements interact with each other, based on their internal structure and placement.
Elements in the periodic table are organized into rows (periods) and columns (groups), each of which corresponds to specific patterns in how their inner particles are distributed. This systematic placement allows scientists to predict the properties of an element based on its position. Elements in the same group typically share similar characteristics because they have a similar configuration of particles in their outermost regions.
Group and Period Trends
As you move across the table, both horizontally and vertically, several trends can be observed in the properties of elements. The placement within a period or group directly correlates with the distribution of particles and their energy states. Some key trends include:
- Group Similarities: Elements within the same vertical column exhibit similar chemical behaviors. This is because they have a similar number of particles in their outermost regions, which governs their chemical bonding and reactivity.
- Period Changes: As you move across a period (left to right), the number of particles increases, leading to changes in atomic size, ionization energy, and electronegativity. These changes are influenced by the way particles fill the available energy levels.
- Transition Elements: Transition elements, located in the middle of the table, have unique arrangements of particles in their sublevels, which leads to distinct characteristics like the ability to form complex ions and exhibit variable oxidation states.
Impact of Electron Placement on Properties
The placement of particles within specific energy levels and sublevels significantly impacts the element’s physical and chemical properties. Key factors affected by this placement include:
- Reactivity: Elements with similar outer configurations are likely to react in similar ways. For example, alkali metals in Group 1 are highly reactive due to their single particle in the outermost region, which they tend to lose easily.
- Bonding Behavior: The configuration of particles determines the types of bonds an element can form. For example, elements with nearly full or nearly empty outer regions often form ionic bonds by gaining or losing particles.
- Atomic Radius: The number of particles and how they are arranged influence the size of the atom. As you move across a period, the atomic radius generally decreases because the particles are drawn closer to the nucleus due to increased nuclear charge.
By understanding the periodic table and how fundamental particles are placed within elements, scientists can predict behaviors, design new materials, and develop technologies that leverage these properties.
Key Concepts of Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that describes the behavior of matter and energy on extremely small scales, where classical physics no longer applies. It provides insights into the nature of particles, their interactions, and the principles that govern them. Central to quantum mechanics is the idea that physical systems do not have definite positions or velocities until they are measured, and that their properties are probabilistic rather than deterministic. This shift from classical concepts of predictability to the probabilistic nature of quantum phenomena has profound implications for understanding the microscopic world.
The principles of quantum mechanics challenge our conventional understanding of reality, offering a framework in which particles can exist in multiple states simultaneously, and their behavior can only be predicted in terms of probabilities. These concepts have transformed our approach to physics, chemistry, and technology, leading to innovations such as semiconductors, lasers, and quantum computing.
Wave-Particle Duality

One of the most important ideas in quantum mechanics is wave-particle duality, which suggests that all particles exhibit both wave-like and particle-like behavior. Depending on the experiment, particles such as light can behave like waves, demonstrating phenomena like interference and diffraction. In other situations, they can act like particles, displaying properties such as discrete impacts. This dual nature is fundamental to understanding how microscopic objects interact with their environment and has been confirmed in numerous experiments.
Uncertainty Principle
Another cornerstone of quantum theory is Heisenberg’s uncertainty principle, which states that it is impossible to simultaneously know both the position and momentum of a particle with absolute precision. The more accurately one of these properties is measured, the less accurately the other can be determined. This inherent limitation is not due to flaws in measurement tools but reflects a fundamental aspect of reality at the quantum level. The uncertainty principle highlights the limits of our knowledge about the behavior of microscopic systems and emphasizes the probabilistic nature of quantum mechanics.
These and other principles of quantum mechanics form the foundation of modern physics, shaping our understanding of everything from the behavior of subatomic particles to the fundamental forces of the universe. Quantum mechanics continues to challenge our perception of reality, offering new perspectives and exciting possibilities in the world of science and technology.
Common Misconceptions in Atomic Theory
The study of the microscopic world has led to the development of various theories that explain the behavior of matter at its most fundamental level. However, some of these ideas are often misunderstood, leading to misconceptions about the nature of particles, their interactions, and the fundamental forces that govern them. It is important to clear up these misunderstandings to foster a deeper and more accurate understanding of the subject.
One common misconception is that the ideas from classical physics apply directly to the behavior of very small particles. However, at the microscopic level, the rules of classical mechanics no longer hold true, and instead, a set of entirely different principles must be used. For instance, the idea of particles having a fixed position and velocity is replaced by probabilities and wave-like behaviors.
Misconception 1: Particles are like mini-solar systems
- One widespread misconception is the analogy of an atom as a tiny solar system, with a central nucleus and orbiting “planets” representing particles. While this model was useful in its time, it doesn’t accurately represent how particles behave at the quantum level.
- In reality, particles do not move in well-defined paths but instead exist in regions of space defined by probabilities. These regions are described as “orbitals,” not fixed orbits.
Misconception 2: The behavior of particles can be precisely predicted
- Another common misunderstanding is that the position and momentum of particles can be determined with complete certainty. In fact, quantum mechanics shows that there is a fundamental limit to how precisely we can know both the position and velocity of a particle at the same time, known as the uncertainty principle.
- This limitation is not due to technological constraints but is inherent to the nature of particles themselves, challenging the deterministic view of classical physics.
By understanding these common misconceptions and embracing the true principles of quantum mechanics, we can gain a more accurate and comprehensive understanding of the microscopic world and the forces that govern it. These shifts in perspective have not only advanced our knowledge of science but have also paved the way for technological innovations that continue to shape our world today.