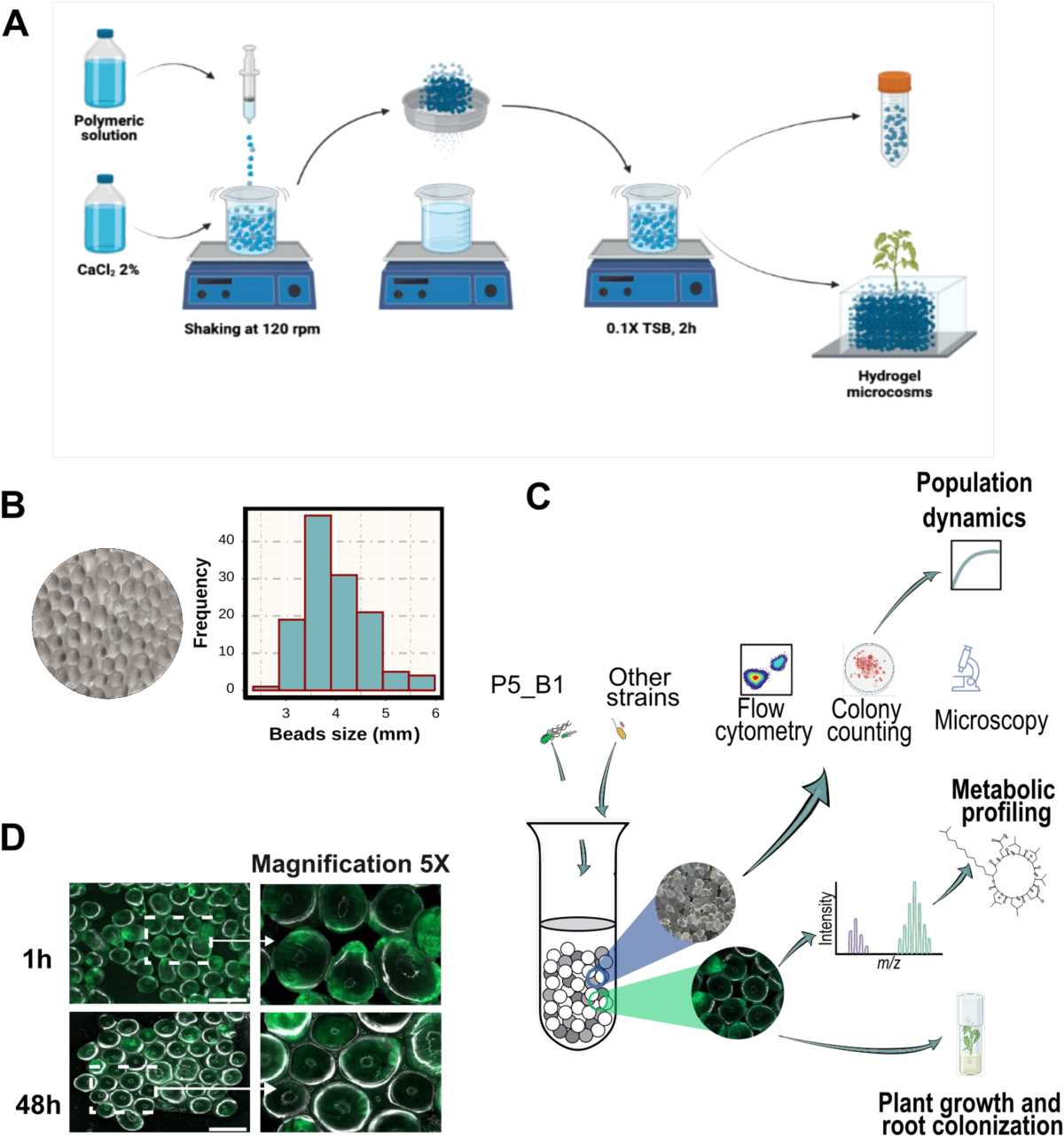
In this section, we delve into the core principles that shape the physical world around us. Understanding how substances behave and transform is essential for anyone looking to grasp the underlying mechanisms of natural processes. From the smallest particles to the grandest reactions, the knowledge gained here serves as the foundation for more complex scientific topics.
By breaking down essential principles, we aim to simplify challenging ideas and provide clarity on how different elements interact with each other. This approach helps in visualizing abstract concepts and provides a deeper insight into the nature of scientific phenomena.
Critical thinking plays a vital role in interpreting these ideas accurately. With the right tools and techniques, the journey through this subject becomes a structured process of discovery and understanding. Each concept builds upon the last, leading to a comprehensive understanding of the world we live in.
Chemistry 10.6 Study of Matter Key
This section explores the fundamental principles behind the interactions and transformations of substances. By examining their composition and behavior under various conditions, we gain a deeper understanding of how different elements and compounds react. The goal is to help learners connect theoretical knowledge with real-world applications, building a solid foundation for more advanced topics in the field.
Understanding the Foundations of Substance Behavior
At the heart of this exploration lies an understanding of how different materials change, interact, and form new compounds. These transformations are governed by underlying forces that dictate the properties of substances. A key aspect is how the physical and chemical properties of these substances affect their reactions. By mastering these principles, students can solve complex problems and predict outcomes with greater accuracy.
Practical Applications in Real-World Scenarios
The practical applications of these principles extend far beyond the classroom. Industries such as pharmaceuticals, manufacturing, and environmental science rely heavily on understanding how substances behave in various contexts. Whether it’s designing new materials or developing innovative processes, the knowledge gained here plays a critical role in technological advancements.
Critical analysis and problem-solving are essential skills when tackling these complex topics. By breaking down each concept into manageable pieces, learners can gain confidence in their ability to apply these concepts in different scientific and real-world situations.
Understanding the Basics of Matter
Grasping the core principles that govern the behavior of substances is essential for building a solid foundation in science. This involves learning how materials are structured, how they interact with one another, and how they can undergo various changes under different conditions. Mastering these fundamental concepts allows us to better comprehend the physical world and its processes.
The Building Blocks of Substances
All substances are made up of tiny particles that have unique properties. Understanding these particles is key to understanding the behavior of materials. Here are the fundamental components:
- Atoms: The basic units of matter that make up everything around us.
- Molecules: Groups of atoms bonded together to form larger structures.
- Elements: Pure substances made up of only one type of atom.
- Compounds: Substances formed by the combination of different elements in fixed ratios.
Key Properties of Substances
The way substances behave can be predicted by their physical and chemical properties. These properties play a crucial role in understanding reactions and transformations. Some of the most important properties include:
- Density: The mass of a substance per unit volume, which determines whether it will float or sink in a liquid.
- Solubility: The ability of a substance to dissolve in another, often forming a homogeneous solution.
- Reactivity: How easily a substance undergoes chemical reactions with other substances.
- Melting and Boiling Points: The temperatures at which a substance changes state from solid to liquid or liquid to gas.
By understanding these properties, we can predict how substances will interact in different environments and under various conditions. This knowledge is essential for practical applications, from creating new materials to understanding natural phenomena.
Key Concepts in Matter Structure
Understanding the fundamental organization of substances is essential for analyzing how they behave in different conditions. The structure of a material determines its properties, how it reacts to external forces, and how it can change form under various circumstances. Grasping these concepts provides a solid foundation for deeper exploration into the various transformations substances undergo.
The Arrangement of Particles
At the most basic level, all substances are made up of particles that are arranged in specific patterns. The way these particles are organized directly impacts the physical properties of the substance. The key aspects of particle arrangement include:
- Atomic Structure: The arrangement of protons, neutrons, and electrons within an atom, determining its chemical behavior.
- Molecular Bonding: The way atoms are held together through chemical bonds, forming molecules with distinct characteristics.
- Crystal Lattices: In solids, atoms or molecules can be arranged in repetitive patterns, influencing the material’s strength and other mechanical properties.
Types of Interactions Between Particles
Particles in different substances interact in various ways, leading to diverse properties and behaviors. These interactions include:
- Van der Waals Forces: Weak attractions between molecules that influence the physical properties of liquids and gases.
- Covalent Bonds: Strong bonds where atoms share electrons, creating stable structures like water and organic compounds.
- Ionic Bonds: Formed when electrons are transferred between atoms, resulting in oppositely charged ions that attract each other.
These interactions are critical for understanding how substances form, react, and change. By exploring these concepts, one can predict and explain the behavior of materials in various contexts, from everyday applications to advanced scientific research.
The Importance of Chemistry in Daily Life
Every aspect of our daily lives is influenced by the principles that govern how substances interact and transform. From the food we eat to the products we use, the natural world is shaped by processes that are deeply rooted in scientific understanding. Recognizing the importance of these principles helps us appreciate the complexity of even the simplest tasks we perform every day.
Everyday Applications

The effects of scientific concepts are seen in countless aspects of daily life. Whether we are cooking, cleaning, or using personal care products, substances undergo reactions that impact their effectiveness and safety. Some common examples include:
- Food Preparation: Chemical reactions like caramelization and fermentation are crucial for cooking and food preservation.
- Cleaning Products: Detergents and soaps work through chemical processes to break down dirt, oil, and stains.
- Medicine: Pharmaceuticals rely on the understanding of reactions within the body to treat various conditions and maintain health.
Environmental Impact and Sustainability
Understanding how substances interact with the environment is key to addressing sustainability challenges. From reducing waste to improving energy efficiency, science helps us find ways to minimize our impact. Important areas include:
- Renewable Energy: Technologies that harness natural resources like wind and solar power depend on principles of energy transformation.
- Recycling: Reusing materials is made possible by chemical processes that break down and repurpose products.
- Pollution Control: Understanding how pollutants affect ecosystems leads to the development of solutions for cleaner air, water, and soil.
These applications highlight how essential scientific knowledge is to improving the quality of life and fostering a more sustainable future for everyone.
How Matter Changes Through Reactions
Substances undergo significant transformations when they interact under specific conditions. These changes can be observed in various forms, such as when a material alters its physical appearance, composition, or even its fundamental properties. By understanding how different substances react with each other, we can predict the outcomes of these processes and apply them in practical scenarios.
Types of Reactions and Their Effects
Reactions can be classified into several categories, each with its own set of characteristics and outcomes. Whether it’s a simple physical change or a more complex chemical transformation, these processes are vital to how substances evolve. The key types of reactions include:
| Reaction Type | Description | Example |
|---|---|---|
| Physical Changes | Changes in the form or state of a substance without altering its chemical composition. | Melting ice into water |
| Chemical Reactions | Substances react to form new compounds with different properties. | Rusting of iron |
| Exothermic Reactions | Reactions that release energy, typically in the form of heat. | Burning wood |
| Endothermic Reactions | Reactions that absorb energy, usually in the form of heat. | Photosynthesis in plants |
Factors Influencing Reactions
The rate and outcome of a reaction can be influenced by several factors. These include:
- Temperature: Higher temperatures generally increase the speed of reactions.
- Concentration: The higher the concentration of reactants, the faster the reaction tends to occur.
- Surface Area: Smaller particle sizes allow for more frequent collisions between molecules, accelerating the reaction.
- Catalysts: Substances that speed up reactions without being consumed in the process.
These factors play a crucial role in determining the efficiency and direction of reactions. By controlling these variables, it’s possible to optimize reactions for industrial, environmental, and everyday applications.
Exploring Atomic and Molecular Properties
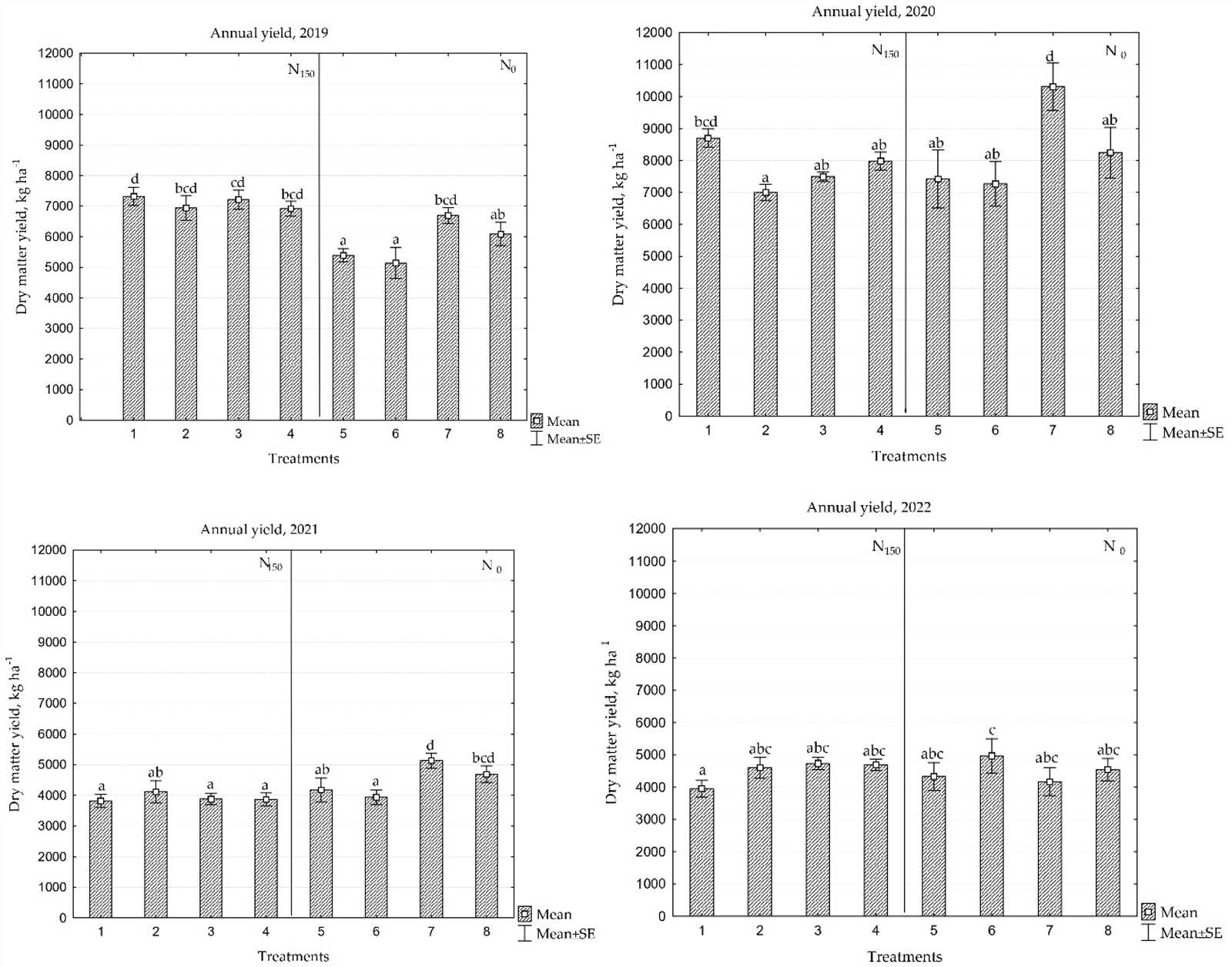
Understanding the fundamental characteristics of the smallest building blocks of matter is essential for explaining the behavior of substances. Atoms and molecules determine how materials interact with each other, how they bond, and the properties they exhibit in different environments. By delving into their structure and behavior, we can gain insights into the wide range of phenomena we observe in the world around us.
Key Atomic and Molecular Features
Atoms and molecules possess unique features that influence their interactions and reactions. These characteristics not only define how substances are structured but also how they behave under various conditions. Some of the primary properties include:
| Property | Description | Example |
|---|---|---|
| Atomic Number | The number of protons in an atom’s nucleus, defining the element. | Carbon has an atomic number of 6. |
| Electronegativity | The ability of an atom to attract electrons in a chemical bond. | Fluorine has the highest electronegativity. |
| Bonding Type | The way atoms are connected, influencing material properties. | Covalent, ionic, and metallic bonds. |
| Molecular Shape | The three-dimensional arrangement of atoms in a molecule. | Water has a bent molecular shape. |
The Role of Electrons in Behavior
Electrons play a crucial role in determining the reactivity and stability of atoms and molecules. Their arrangement in various energy levels influences how substances interact with one another. Understanding electron configurations helps explain why certain materials are reactive while others are inert. Additionally, it explains how atoms form bonds, create compounds, and engage in chemical reactions.
By exploring these properties, scientists can predict how substances will behave, react, and change in different conditions. This knowledge is fundamental not only in scientific research but also in developing new materials and technologies for everyday use.
Explaining Physical vs Chemical Changes
Substances undergo various transformations that can either be temporary or permanent. These changes are classified into two broad categories: physical and chemical. While both types of changes alter the appearance or structure of a substance, they differ fundamentally in the way the substance itself is affected. Understanding these distinctions is crucial for interpreting how materials behave in different conditions.
Physical Changes
Physical changes refer to alterations that affect a substance’s form or state without changing its chemical composition. These transformations are typically reversible and involve changes in physical properties such as size, shape, or phase. Common examples include:
- Melting: The process of a solid turning into a liquid.
- Boiling: The transition from liquid to gas due to heat.
- Shaping: Altering the form of a substance, like bending or cutting it.
- Dissolving: When a solid dissolves in a liquid without forming a new substance, like sugar in water.
In all these cases, the substance remains the same at a molecular level; only its physical state or appearance is altered.
Chemical Changes
Chemical changes, on the other hand, involve a transformation that results in the creation of one or more new substances with different properties. These changes are usually not reversible under normal conditions and often require energy input or a specific environment. Some examples of chemical changes include:
- Burning: Combustion of wood that produces ash and gases.
- Rusting: Iron reacting with oxygen to form iron oxide.
- Digestion: The chemical breakdown of food in the stomach.
- Fermentation: Yeast converting sugars into alcohol and carbon dioxide.
In these cases, the substance’s molecular structure changes, forming new compounds with different characteristics.
Recognizing whether a change is physical or chemical helps explain how substances interact and transform in the natural world, from everyday occurrences to complex industrial processes.
The Role of Energy in Chemical Processes
Energy plays a fundamental role in driving the transformations that occur during reactions. Whether substances are breaking apart or combining, the changes often require or release energy in various forms. Understanding how energy interacts with matter during these processes is crucial for explaining the direction and rate of reactions. From heat to light, energy influences how and why reactions take place, as well as how much energy is needed or released in the process.
Types of Energy Involved in Reactions
During chemical reactions, energy can be absorbed or released depending on the nature of the process. The two primary forms of energy involved in these changes are:
| Energy Type | Description | Example |
|---|---|---|
| Exothermic Energy | Energy is released into the surroundings, usually in the form of heat or light. | The combustion of fuel in a car engine. |
| Endothermic Energy | Energy is absorbed from the surroundings, often in the form of heat. | Photosynthesis in plants, where energy from sunlight is absorbed. |
The Importance of Activation Energy

All reactions require an initial input of energy to begin, known as activation energy. This is the energy needed to break the bonds in the reactants so that new bonds can form. Even in exothermic reactions, activation energy is essential to get the reaction started. In industrial applications, controlling activation energy can help optimize reaction rates and efficiency.
The way energy is managed during these transformations can determine the efficiency and outcome of a process. Whether energy is released or absorbed, understanding its role helps to manipulate reactions for desired results, from industrial manufacturing to biological processes.
Identifying Elements and Compounds
Understanding the distinction between simple substances and complex mixtures is essential in the world of materials. Elements are the fundamental building blocks of all substances, while compounds are combinations of two or more elements that are chemically bonded. Recognizing and distinguishing between these two forms allows scientists and engineers to manipulate and utilize materials more effectively. The process of identification is key in various fields, from manufacturing to healthcare, where knowing the exact composition of a substance can determine its use or safety.
Elements are pure substances that cannot be broken down into simpler substances by ordinary chemical means. They are represented by symbols on the periodic table, each corresponding to a specific type of atom. Some well-known examples of elements include oxygen (O), carbon (C), and gold (Au).
In contrast, compounds are substances formed when two or more elements combine chemically. These compounds exhibit unique properties that differ from those of the individual elements that make them up. For instance, water (H2O) is a compound composed of hydrogen and oxygen, but it has entirely different properties from either of the two elements on their own.
The identification of elements and compounds relies on various methods, including:
- Chemical Analysis: Techniques such as spectroscopy, chromatography, and titration help identify the composition of a substance.
- Physical Properties: Properties like boiling point, melting point, and density can help distinguish between different elements and compounds.
- Reactivity Tests: Observing how a substance reacts with other chemicals can indicate whether it is an element or a compound.
By understanding these distinctions, it becomes easier to predict how substances will behave in different reactions, allowing for better control over industrial and scientific processes.
How to Solve Matter-Related Problems
Solving problems involving the transformation and behavior of substances requires a methodical approach. Whether the task is identifying the composition of a substance, understanding its properties, or predicting how it will react under certain conditions, breaking the problem down into manageable steps is crucial. A systematic process helps to ensure accurate results and a deeper understanding of how substances interact.
Step 1: Understand the Problem
The first step in solving any scientific problem is to thoroughly read and understand the problem at hand. This involves identifying what is being asked, what information is provided, and what is needed to reach a solution. Clarifying the conditions or constraints in the problem will guide the selection of the appropriate methods or formulas.
Step 2: Identify Relevant Concepts and Tools
Once the problem is clear, it’s important to identify the concepts and tools required to solve it. This may include recognizing the physical or chemical properties involved, determining the relationships between different variables, or selecting the right calculations. Common tools used for these types of problems include:
- Dimensional Analysis: This method is useful for converting units and ensuring that calculations are consistent.
- Stoichiometry: A key technique for solving problems involving reactants and products in chemical reactions.
- Formula Derivations: Using established equations, such as the ideal gas law, to solve for unknown variables.
By understanding the principles behind these tools, one can apply them effectively to solve problems involving substances and their interactions.
Once all the necessary concepts and methods are identified, the next step is to carry out the calculations or observations carefully, keeping track of units and significant figures to ensure accuracy.
Analyzing the Behavior of Gases
Understanding how gases behave is essential for a wide range of scientific and industrial applications. Gases differ from solids and liquids in that they are highly compressible and their particles are far apart. Their behavior is influenced by various factors, such as pressure, volume, and temperature. By analyzing these factors, scientists can predict how gases will react under different conditions, which is crucial for everything from weather forecasting to designing engines and industrial processes.
Key Factors Affecting Gas Behavior
The behavior of gases can be described by several fundamental principles and laws. These help scientists and engineers understand how gases respond to changes in their environment. The main factors that influence gas behavior include:
- Pressure: The force that gas molecules exert when they collide with the walls of their container. Increasing pressure typically decreases volume, assuming temperature is constant.
- Volume: The space a gas occupies. If the volume is reduced, the particles are compressed, which can increase pressure or temperature.
- Temperature: The average kinetic energy of gas molecules. When temperature increases, the particles move faster, often leading to increased pressure or volume if the other factors remain constant.
- Amount of Gas: The number of gas particles. More particles will result in greater pressure, assuming volume and temperature are unchanged.
Common Gas Laws
Several gas laws describe the relationships between these factors. These laws provide insight into how gases behave under various conditions:
- Boyle’s Law: Describes the inverse relationship between pressure and volume. As pressure increases, volume decreases, provided the temperature remains constant.
- Charles’s Law: Explains the direct relationship between volume and temperature. As temperature increases, the volume of the gas increases if pressure is held constant.
- Avogadro’s Law: States that the volume of a gas is directly proportional to the number of gas particles, assuming pressure and temperature are constant.
By applying these laws, scientists can predict how gases will behave in various scenarios, making it possible to design systems that efficiently utilize gas properties for specific applications, such as engines, refrigeration, and chemical reactions.
The Law of Conservation of Mass
The principle that mass is neither created nor destroyed during a chemical or physical process is fundamental to understanding how substances interact and transform. This law forms the basis for many scientific concepts and is critical in ensuring the accuracy of experiments, especially when dealing with reactions and energy changes. In essence, the mass of the reactants in a closed system will always equal the mass of the products, as no mass is lost or gained in the process.
Understanding the Concept
The Law of Conservation of Mass is a cornerstone of classical science, and its implications extend to numerous fields, including physics, biology, and engineering. It asserts that when substances undergo transformation–whether through chemical reactions, phase changes, or mechanical processes–the total mass remains unchanged, provided the system is closed and no external factors influence the process.
- In a Chemical Reaction: The total mass of the reactants will always equal the total mass of the products. This is a fundamental concept in stoichiometry.
- In Physical Changes: Even when a substance changes state, such as from solid to liquid or gas, the mass remains the same, as no particles are lost or gained in the process.
- In Isolated Systems: Mass conservation holds true in a closed system where no material enters or exits, ensuring that the mass before and after the reaction is identical.
Applications of Mass Conservation
In practical terms, this principle is used to balance chemical equations and ensure that reactions are properly understood. Here are some ways it is applied:
- Balancing Chemical Equations: To create accurate equations, it is essential that the mass of the reactants equals the mass of the products.
- Industrial Applications: In chemical manufacturing, ensuring mass conservation helps optimize the use of raw materials and energy.
- Environmental Science: The principle aids in understanding and minimizing waste in industrial processes, ensuring that materials are efficiently recycled or transformed.
By adhering to this law, scientists can predict the outcomes of reactions with a high degree of precision, allowing for more controlled and efficient processes in various fields.
Properties of Solids Liquids and Gases
The different phases of substances–solids, liquids, and gases–possess distinct properties that govern their behavior under various conditions. These states of matter differ in their structural arrangements, energy levels, and the ability of particles to move. Understanding these characteristics is fundamental to explaining the physical properties and transformations substances undergo during processes such as heating, cooling, and mixing.
Solids
In a solid state, particles are tightly packed and only vibrate in place, resulting in a fixed shape and volume. The rigidity of solids allows them to maintain their form unless subjected to external forces that may cause deformation or breakage. These substances have a high degree of order in their internal structure, contributing to their stability and resistance to flow or compression.
- Fixed Shape: Solids maintain their shape and do not flow.
- Fixed Volume: The volume of a solid remains constant.
- High Density: Solids typically have a higher density than liquids and gases due to the close packing of particles.
Liquids
Liquids possess a definite volume but no fixed shape. Instead, they adapt to the shape of their container. The particles in liquids are not as tightly bound as in solids, allowing them to flow and move past each other. This increased mobility allows liquids to take the form of their container, while still maintaining their volume.
- Fluidity: Liquids can flow and change shape, but their volume remains constant.
- Indefinite Shape: Liquids take the shape of the container they occupy.
- Moderate Density: Liquids generally have a lower density than solids but higher than gases.
Gases
Gases have neither a fixed shape nor volume. The particles in gases are widely spaced and move freely at high speeds, resulting in their ability to expand and fill any container. This lack of structural rigidity means gases are highly compressible and can spread out to occupy large volumes, given enough space and the right conditions.
- No Fixed Shape or Volume: Gases spread to fill the shape and volume of any container.
- Low Density: Gases have a much lower density compared to solids and liquids.
- High Compressibility: Gases can be compressed easily due to the large distances between particles.
In summary, solids, liquids, and gases represent the three most common phases of substances, each with distinct physical characteristics. Understanding these differences is crucial for explaining how substances behave and change under different conditions, whether in natural processes or industrial applications.
Applications of Matter in Technology
The properties and behaviors of different substances play a pivotal role in the development of modern technologies. From the materials used in electronics to the advanced processes in medicine and engineering, understanding the behavior of substances under various conditions is essential for creating new and innovative technologies. By manipulating the characteristics of different compounds and elements, engineers and scientists are able to design products that enhance everyday life and push the boundaries of what is possible.
Materials in Electronics
In the world of electronics, the behavior of materials such as metals, semiconductors, and insulators is crucial. These substances are selected based on their ability to conduct electricity, resist heat, and interact with other elements in specific ways. For example, silicon is widely used in the production of microchips, which are the heart of modern computers, phones, and other devices.
- Conductivity: Metals like copper are used for wiring due to their excellent conductivity, allowing electricity to flow efficiently.
- Semiconductors: Silicon and germanium are used in transistors, which regulate the flow of electrical current in devices.
- Insulators: Materials like rubber and plastic prevent the unwanted flow of electricity, ensuring safety in electronic systems.
Innovations in Medicine
The application of materials in medicine has led to life-saving innovations. From drug delivery systems to medical implants, the manipulation of materials allows for improved health outcomes. For example, biocompatible materials, which do not cause adverse reactions in the body, are used in prosthetics, heart valves, and surgical implants.
- Drug Delivery Systems: Polymers are used to create controlled-release capsules, ensuring that medication is delivered in the right amounts at the right times.
- Biocompatible Implants: Materials like titanium are used in medical implants because they integrate well with the body without causing harm.
- Diagnostic Tools: Materials like specialized films and sensors are used in imaging technologies such as MRI and X-rays, improving diagnostic accuracy.
Environmental Applications

The understanding of substance properties has also led to advances in environmental technology. Materials that can purify water, capture carbon dioxide, or generate clean energy are crucial in combating pollution and climate change. For instance, solar panels utilize semiconductors to convert sunlight into electricity, providing a sustainable source of energy.
- Water Filtration: Membranes made from advanced materials can remove toxins and impurities from water, ensuring clean drinking water in many parts of the world.
- Carbon Capture: New materials are being developed to capture CO2 from the atmosphere, helping to mitigate the effects of global warming.
- Renewable Energy: Solar cells, wind turbines, and other renewable technologies rely on materials that harness natural forces to generate electricity efficiently.
In conclusion, the practical uses of different substances are integral to the progress of technology across multiple industries. From electronics to medicine and environmental protection, the ability to understand and manipulate materials has led to groundbreaking innovations that continue to shape the future.
Practical Tips for Chemistry Students
For those pursuing a deep understanding of how substances interact and transform, practical approaches to learning can make a significant difference in mastering complex concepts. Success in this field requires more than just memorization–it involves developing problem-solving skills, critical thinking, and a solid grasp of the foundational principles that govern the behavior of different elements and compounds. Here are several strategies that can help students excel in their academic journey.
1. Master the Basics
A solid foundation in fundamental principles is essential for tackling more advanced topics. Ensure that you have a firm understanding of key concepts such as atomic structure, chemical bonding, and the properties of various substances. These basics serve as building blocks for more complex ideas, making it easier to grasp new material as you progress in your studies.
- Review regularly: Revisit core topics often to reinforce your understanding.
- Ask questions: Don’t hesitate to ask your instructors or peers when something isn’t clear.
- Practice problems: Regular problem-solving will help you internalize concepts and apply them in real-world scenarios.
2. Get Hands-On Experience
Practical lab work is an integral part of learning. Engaging in experiments allows students to see theory in action and develop a better understanding of chemical processes. By following procedures carefully and observing reactions firsthand, students gain invaluable insights into how substances behave under different conditions.
- Pay attention to safety: Always follow safety guidelines and wear protective equipment during experiments.
- Record observations: Keep detailed notes on each experiment–these will be invaluable when reviewing your results and writing reports.
- Reflect on results: After conducting experiments, take time to analyze and discuss your findings with classmates or instructors.
3. Utilize Visual Aids and Resources
Visual tools such as diagrams, charts, and molecular models can help you better understand complex reactions and structures. Many students find that they can retain information more effectively when they can visualize concepts. Additionally, online resources and textbooks often provide alternative explanations that might resonate with different learning styles.
- Diagrams: Draw structures and processes to visualize how elements and compounds interact.
- Online tutorials: Take advantage of educational videos and interactive simulations to reinforce concepts.
- Flashcards: Use flashcards to memorize key terms and equations for quicker recall during exams.
By implementing these practical tips, students can approach their studies with confidence and develop a deeper understanding of the subject. Consistency, hands-on practice, and utilizing multiple learning tools will not only improve academic performance but also help develop critical skills that are valuable for future careers in the field.