
In the world of science, understanding how substances interact, transform, and behave under different conditions is essential. The exploration of these concepts allows us to explain a wide variety of phenomena, from the smallest particles to large-scale reactions that shape our daily lives. Mastery of these principles requires a solid grasp of the fundamental processes that govern their behavior and structure.
The section offers a detailed guide to help students and enthusiasts navigate complex questions and problems. With step-by-step instructions and clear examples, it provides clarity on how to tackle and analyze different scenarios. Emphasis is placed on logical thinking, problem-solving techniques, and effective strategies to comprehend challenging topics.
Through engaging exercises and illustrative examples, the guide serves as a useful tool for reinforcing key concepts. Whether you are preparing for exams or simply wish to strengthen your understanding, the insights presented here will enhance your knowledge and confidence in the subject matter.
Chemistry A Study of Matter 3.6 Answer Key
In this section, we will dive into the detailed breakdown of the scientific principles covered in the previous lesson. By focusing on the core concepts and providing thorough solutions to the exercises, this guide helps clarify how different substances react, combine, and transform. Each step is carefully explained to ensure a deep understanding of the fundamental processes at play.
Through a series of practical examples, you’ll gain insight into how to approach complex problems and develop effective strategies for solving them. The solutions provided are designed to enhance your comprehension, allowing you to apply the principles in various real-world situations. Step-by-step methods are outlined to facilitate learning and ensure mastery of the material.
The goal is to equip you with the tools needed to confidently navigate challenges in the field. By breaking down each problem and explaining the reasoning behind the solutions, this section encourages a comprehensive grasp of the subject matter. Understanding the reasoning behind each answer is key to mastering the concepts and excelling in future applications.
Understanding the Basics of Matter
The fundamental building blocks of everything around us are essential to grasping the complexities of how substances interact and behave. These core principles form the foundation for more advanced topics and offer a clear framework for understanding the physical world. By studying these concepts, we learn how elements and compounds come together, change, and respond to various forces.
At the heart of this understanding is the behavior of atoms and molecules. The way they bond, move, and react with one another determines the properties of substances and how they behave under different conditions. By exploring these interactions, we begin to unravel the mysteries of nature, from the smallest particles to larger, more visible transformations.
As we explore the essentials of how substances are structured and interact, it becomes clear that mastering these basic concepts is crucial for further study in any scientific discipline. The deeper knowledge of how things are formed and altered enables us to predict outcomes, solve problems, and apply these insights to practical scenarios in both the lab and everyday life.
Key Concepts in Chemistry 3.6
Understanding the fundamental principles behind the behavior of substances is essential for mastering any scientific discipline. This section focuses on the core ideas that underpin the processes of transformation and interaction. Grasping these concepts provides the necessary foundation for further exploration and practical application in various scientific fields.
The key topics explored here cover the different ways elements and compounds behave under specific conditions. By examining their properties, we can predict reactions, determine outcomes, and better understand the natural world. A solid understanding of these principles will aid in solving complex problems and applying scientific knowledge to real-world scenarios.
| Concept | Description |
|---|---|
| Atomic Structure | The arrangement of protons, neutrons, and electrons in an atom and how this affects its properties. |
| Chemical Bonds | The forces that hold atoms together in molecules, including ionic, covalent, and metallic bonds. |
| States of Matter | The physical forms (solid, liquid, gas) in which substances exist and how they change under different conditions. |
| Energy Transfer | How energy is absorbed or released during chemical reactions and physical changes. |
| Reactivity | The tendency of a substance to undergo a chemical change when exposed to other substances. |
How to Approach Chemistry Problems
Tackling complex scientific problems requires a systematic approach that breaks down each challenge into manageable steps. By understanding the underlying principles and carefully analyzing the information provided, it’s possible to find effective solutions to even the most intricate questions. Developing a clear strategy ensures that all aspects of the problem are addressed logically and thoroughly.
Step-by-Step Problem Solving
A well-structured approach involves several key steps to ensure accuracy and efficiency. Start by identifying the problem and gathering all relevant information. Then, work through the equations or concepts systematically, applying the appropriate techniques and formulas. Finally, double-check the results to confirm that they align with the expected outcomes.
Common Strategies for Success
Various strategies can be applied to solve scientific problems, depending on the type of question. Whether you’re working with formulas, diagrams, or conceptual explanations, using a consistent method will help you stay organized and focused. Here’s an overview of some commonly used strategies:
| Strategy | Description |
|---|---|
| Identify Key Information | Carefully extract the essential data from the problem statement, focusing on numbers, units, and variables. |
| Draw Diagrams or Models | Visual aids can simplify complex relationships and help clarify difficult concepts. |
| Apply Relevant Equations | Use established formulas and principles to relate the given information and solve for unknowns. |
| Verify Units and Conversions | Ensure that all units are consistent and correctly converted to avoid errors in calculations. |
| Check Final Answer | Review the answer for reasonableness, ensuring it aligns with expectations and logical consistency. |
Step-by-Step Guide to Answering
When solving complex scientific problems, breaking them down into smaller, more manageable tasks is essential. This structured approach ensures that no important steps are overlooked and that each component is addressed systematically. By following a clear process, you can efficiently work through any challenge, making sure every concept is fully understood and applied correctly.
The first step is to carefully read the problem and identify the key details. Understanding what is being asked and what information is provided sets the foundation for the solution. Afterward, you should organize your thoughts and determine the best method for tackling the problem, whether it involves calculations, conceptual reasoning, or a combination of both.
Next, proceed with applying the appropriate formulas or techniques, ensuring all variables are properly accounted for. As you work through the solution, it’s crucial to maintain a logical flow and double-check your steps. Final verification of your results is key to confirming the accuracy of your work.
Breaking Down Chemical Reactions
Understanding the processes that occur during a reaction is crucial to mastering the dynamics of how substances transform into new compounds. These transformations are governed by specific rules and principles that dictate how atoms and molecules interact with one another. By breaking down these processes into individual steps, we can better understand the underlying mechanisms and predict the outcomes of various reactions.
Identifying Reactants and Products
The first step in analyzing a reaction is to identify the reactants and products involved. Reactants are the substances that undergo change, while products are the new substances formed. By recognizing these components, we can gain insight into the direction and nature of the reaction. Understanding how the atoms are rearranged helps explain the changes observed in the physical properties of the substances.
Balancing Reactions
Another key aspect of analyzing chemical reactions is ensuring that the equation is balanced. This involves adjusting the coefficients of the reactants and products to reflect the law of conservation of mass, which states that matter cannot be created or destroyed. Balancing the equation ensures that the number of atoms for each element is the same on both sides, providing an accurate representation of the reaction.
Exploring Atomic Structures in Detail
At the heart of every substance lies the atomic structure, which determines its properties and behavior. By understanding the composition and arrangement of these tiny particles, we can explain how different elements interact, bond, and form compounds. This section delves into the key aspects of atomic structures, providing a deeper understanding of how atoms form the foundation of all matter.
Atoms consist of several fundamental components, each playing a vital role in their overall function. These particles interact with one another in ways that influence the physical and chemical properties of materials. Understanding their structure is crucial for grasping concepts such as reactivity, stability, and the behavior of different substances under various conditions.
- Protons: Positively charged particles found in the nucleus of an atom. The number of protons determines the element’s identity.
- Neutrons: Neutral particles also located in the nucleus. Neutrons, together with protons, make up the atom’s mass.
- Electrons: Negatively charged particles that orbit the nucleus in defined energy levels or shells.
Each of these components is essential to understanding how atoms behave in different environments. The arrangement of electrons, for instance, dictates how atoms bond with others, while the number of protons influences the atom’s chemical properties. By studying these structures, scientists can predict how elements will interact and form new substances.
- Electron Configuration: The specific arrangement of electrons in an atom’s energy levels, which determines its chemical behavior.
- Valence Electrons: Electrons in the outermost shell, crucial for bonding and determining an element’s reactivity.
- Isotopes: Atoms of the same element with different numbers of neutrons, affecting their mass and stability.
In the next section, we will explore how atomic structures relate to molecular formation and the chemical reactions that govern our world. Understanding these details is essential for making sense of how substances interact and change over time.
Common Mistakes to Avoid in Chemistry
When tackling scientific problems, it’s easy to make errors that can lead to incorrect conclusions or results. These mistakes often stem from a lack of attention to detail or misunderstanding fundamental concepts. Recognizing and avoiding these pitfalls is key to achieving accuracy and improving problem-solving skills in scientific tasks.
Many common errors arise during calculations, where small missteps can cause significant discrepancies. Other mistakes may occur when interpreting data or when applying theoretical concepts incorrectly. To avoid these issues, it’s essential to carefully review each step of the process and verify your understanding of the principles involved.
- Neglecting Units: Failing to include or convert units correctly can lead to inaccurate results. Always check that the units are consistent throughout the calculation.
- Incorrect Formula Application: Using the wrong formula or applying it incorrectly can drastically alter your results. Be sure to choose the appropriate equation for each situation.
- Overlooking Significant Figures: Ignoring the correct number of significant figures can affect the precision of your answer. Pay attention to the level of accuracy required by the problem.
- Misinterpreting Data: Data can often be misread or misunderstood, leading to errors in conclusions. Double-check measurements and results for accuracy before proceeding.
- Skipping Logical Steps: Rushing through problems or skipping steps can result in missing key insights or calculations. Always follow a step-by-step approach to ensure accuracy.
By being mindful of these common mistakes, you can improve both your problem-solving abilities and your understanding of scientific principles. Regular practice and careful attention to detail are the best ways to avoid errors and build confidence in your abilities.
Practical Applications of Matter Studies
The understanding of the components that make up different substances plays a crucial role in various industries and fields of research. By exploring how atoms and molecules interact, scientists can apply this knowledge to solve real-world problems, create new materials, and develop innovative technologies. These practical applications shape many aspects of our daily lives, from medicine to environmental protection.
In industries ranging from pharmaceuticals to energy production, the manipulation of basic elements and compounds leads to advancements that improve efficiency, sustainability, and human well-being. By understanding how different substances behave under various conditions, researchers can design solutions to everyday challenges.
- Material Science: The development of stronger, lighter, and more durable materials relies on a deep understanding of atomic structures and bonding. These materials are used in everything from electronics to construction.
- Environmental Protection: Knowledge of how pollutants interact with natural systems helps in creating technologies to reduce emissions, purify water, and restore ecosystems.
- Pharmaceuticals: Advances in drug design are driven by insights into molecular structures and reactions. This knowledge is essential for creating more effective treatments and vaccines.
- Energy Production: Understanding the properties of different substances is crucial for developing renewable energy sources, such as solar panels and biofuels, that are both efficient and environmentally friendly.
- Agriculture: Insights into soil composition and plant nutrition help in the development of better fertilizers and pest control methods, improving crop yield and sustainability.
These applications demonstrate the far-reaching impact that the study of the fundamental building blocks of matter has on various sectors. By continuing to explore and manipulate these substances, we unlock the potential for new discoveries that benefit society as a whole.
Techniques for Mastering Chemical Equations
Understanding how to balance and interpret chemical reactions is a foundational skill in science. Mastering the art of writing and solving chemical equations allows for accurate predictions about how substances interact. By following systematic techniques, one can develop a clear approach to handling these reactions and avoid common mistakes.
Each chemical equation represents a relationship between reactants and products, and it’s essential to ensure that the equation follows the law of conservation of mass. This requires balancing the number of atoms on both sides, which can be challenging but is achievable with practice and the right approach.
- Identify Reactants and Products: Begin by clearly identifying the substances involved in the reaction. Knowing the compounds and elements helps in predicting the outcomes and understanding the reaction’s behavior.
- Balance the Elements: Use coefficients to balance the number of atoms of each element on both sides of the equation. Start with the most complex molecules and work your way to the simpler ones.
- Adjust Coefficients, Not Subscripts: Never alter the chemical formulas (subscripts) of the reactants or products. Only adjust the coefficients in front of the compounds to balance the equation.
- Double-Check for Accuracy: After balancing, ensure that the total number of atoms for each element is the same on both sides. Also, verify that the equation is in its simplest form.
- Practice with Different Types of Reactions: Focus on mastering different reaction types–synthesis, decomposition, combustion, etc. Each type has its own patterns and tendencies that can make balancing easier.
With consistent practice and careful attention to detail, mastering chemical equations becomes an easier and more intuitive process. Understanding the underlying principles allows one to approach complex reactions with confidence and accuracy.
Why Molecular Bonding Matters in Chemistry
Understanding how atoms combine to form molecules is essential for grasping how substances interact and behave. The type and strength of the connections between atoms determine a compound’s properties, including its reactivity, state of matter, and overall stability. This knowledge is crucial for developing new materials, pharmaceuticals, and technologies.
Molecular bonding dictates the physical and chemical properties of substances. Whether a substance is a solid, liquid, or gas depends on the way molecules are held together. The nature of these bonds can also influence how substances react with one another, making this a key concept in predicting and controlling chemical behavior.
Types of Bonds
There are several types of bonds that atoms can form, each with its own characteristics and implications for the substance’s properties. The most common types include:
| Bond Type | Characteristics | Examples |
|---|---|---|
| Covalent Bond | Atoms share electrons to fill their outer shells. | Water (H₂O), Oxygen (O₂) |
| Ionic Bond | Electrons are transferred from one atom to another, creating charged particles (ions). | Sodium Chloride (NaCl), Magnesium Oxide (MgO) |
| Metallic Bond | Electrons are free to move throughout the metal structure, creating conductivity. | Copper (Cu), Iron (Fe) |
Importance of Molecular Bonding
Understanding molecular bonding is crucial for several reasons:
- Predicting Behavior: Knowing how atoms bond helps predict how substances will react under different conditions.
- Material Design: By manipulating molecular bonds, scientists can design new materials with desired properties, such as stronger, more durable substances.
- Environmental Impact: Molecular understanding helps develop greener technologies and sustainable materials.
In essence, molecular bonding is the foundation of how substances form, interact, and behave, making it a central concept in many scientific and industrial applications. Without this understanding, advances in technology and medicine would be far more difficult to achieve.
Reviewing Chemical Properties and Changes
Understanding the nature of substances and how they transform under different conditions is essential for predicting reactions and behavior in various environments. Chemical properties describe how a substance interacts with other materials, while chemical changes refer to the process by which one substance is converted into another. These concepts are fundamental in both theoretical and applied sciences, as they help explain everything from industrial processes to biological functions.
Chemical properties are characteristics that determine how a substance will react with others. For instance, reactivity with acids, oxidation states, and flammability are examples of chemical properties. These traits are not immediately observable and often require specific tests to determine. On the other hand, chemical changes involve the actual transformation of one substance into another, resulting in a new set of properties.
- Reactivity with Acids: Some materials, like metals, react with acids to produce hydrogen gas. This reaction is a classic example of a chemical change.
- Combustibility: Substances like wood or gasoline undergo combustion when exposed to oxygen, turning into carbon dioxide and water. This is a typical chemical change associated with energy release.
- Oxidation: The process of oxidation, such as when iron rusts, involves a substance reacting with oxygen in the air to form a new compound, demonstrating a chemical property and change.
Recognizing these properties and understanding how they lead to changes is vital for manipulating substances in laboratories, manufacturing, and even everyday life. This knowledge lays the groundwork for advancements in fields ranging from environmental science to pharmacology.
Explaining States of Matter in Chemistry
The different forms that substances can take–such as solid, liquid, and gas–are essential in understanding how they behave under various conditions. Each state is characterized by unique properties, including the arrangement of particles, energy levels, and how they respond to changes in temperature and pressure. Recognizing these states and their transitions is fundamental for a wide range of scientific applications, from industrial processes to biological systems.
Substances can exist in different states depending on the amount of energy they possess. In each state, the particles–atoms or molecules–interact in distinct ways that determine their physical properties such as shape, volume, and compressibility. The transformation from one state to another occurs through processes like melting, freezing, condensation, and evaporation, which involve energy exchange.
- Solid: In this state, particles are closely packed and vibrate in place. Solids have a fixed shape and volume, and they are not compressible.
- Liquid: Here, particles are still close together but can move around each other. Liquids have a definite volume but take the shape of their container, and they are slightly compressible.
- Gas: In gases, particles are widely spaced and move freely. Gases do not have a fixed shape or volume and are highly compressible.
Changes between these states occur when energy is added or removed. For example, heating a solid can cause it to melt into a liquid, while cooling a gas can condense it into a liquid. Understanding these processes is crucial for explaining natural phenomena, designing materials, and controlling reactions in various scientific fields.
How Energy Affects Matter Transformation
The transformation of substances from one form to another is greatly influenced by the energy they absorb or release. Whether it’s a change in temperature or pressure, the amount of energy a system gains or loses determines the phase and behavior of the material involved. Understanding how energy affects these transformations is crucial for explaining everyday phenomena and industrial processes.
Energy changes cause particles in substances to move faster or slower, which affects their interactions and arrangement. For example, heating a substance generally increases the kinetic energy of its particles, causing them to move more freely and potentially transition to a different state. Conversely, cooling a substance removes energy, causing particles to slow down and change their arrangement.
Energy and Phase Changes
- Melting: When a solid absorbs energy, it gains kinetic energy, causing its particles to vibrate faster and eventually break free from their fixed positions, turning into a liquid.
- Evaporation: Adding energy to a liquid allows the particles to move quickly enough to escape the surface and become gas molecules, a process known as evaporation.
- Condensation: When gas molecules lose energy, their speed decreases, and they come together to form a liquid, a process opposite to evaporation.
- Freezing: In this process, energy is removed from a liquid, causing particles to slow down and arrange themselves into a solid structure.
The Role of Energy in Chemical Reactions
Energy also plays a significant role in chemical reactions. In exothermic reactions, energy is released, often in the form of heat or light, as new bonds are formed. On the other hand, endothermic reactions absorb energy to break existing bonds, requiring an input of energy to proceed. This transfer of energy is a key factor in understanding how and why substances interact to form new compounds.
Understanding the impact of energy on these transformations helps scientists control and manipulate reactions, leading to innovations in fields like manufacturing, energy production, and medicine.
Interactive Exercises for Practice
Engaging with interactive exercises is a powerful way to reinforce concepts and improve your understanding of various scientific principles. These exercises offer an opportunity to test your knowledge, identify areas that need further study, and develop problem-solving skills. Whether you’re solving simple tasks or more complex scenarios, hands-on practice enhances retention and comprehension.
Interactive activities are designed to simulate real-world situations and scientific phenomena. They provide immediate feedback, allowing you to adjust your approach and correct mistakes on the fly. This active learning approach is more effective than passive reading, as it encourages deeper cognitive engagement and critical thinking.
Examples of Interactive Tasks
- Balancing Equations: Practice balancing chemical reactions by entering reactants and products into an interactive tool that checks if your equation is balanced.
- Phase Transition Simulation: Explore how substances change between states by manipulating variables such as temperature and pressure in a virtual environment.
- Reaction Rate Calculator: Experiment with different factors that affect reaction speed, such as concentration, temperature, and catalysts, to observe their effects.
- Energy Transfer Modeling: Use a simulation to model energy transfer during processes like heating, cooling, or phase transitions.
Benefits of Interactive Exercises
- Instant Feedback: Receive immediate results on your progress, helping you understand where corrections are needed.
- Self-Paced Learning: Take control of your learning journey by working through problems at your own pace, revisiting challenging topics as needed.
- Engagement and Motivation: Interactive exercises keep you engaged, making learning more enjoyable and less monotonous.
These interactive exercises are available in various formats, from online quizzes to interactive simulations. Incorporating them into your learning routine can greatly improve both your conceptual understanding and practical application of scientific principles.
Tips for Effective Study of Chemistry
Mastering scientific concepts requires more than just reading through textbooks; it involves developing strategies that engage both your analytical and practical skills. To excel in this field, a combination of effective planning, consistent practice, and active problem-solving is essential. Below are some valuable tips that can help you approach your learning more efficiently and build a deeper understanding of the subject.
Organize and Plan Your Approach
Creating a structured study plan is key to managing the large volume of information you need to absorb. Break down complex topics into smaller, manageable sections and set clear goals for each session. Review your notes regularly to reinforce concepts, and prioritize areas that you find particularly challenging. A disciplined study schedule can help you stay focused and motivated.
Engage in Active Learning Techniques
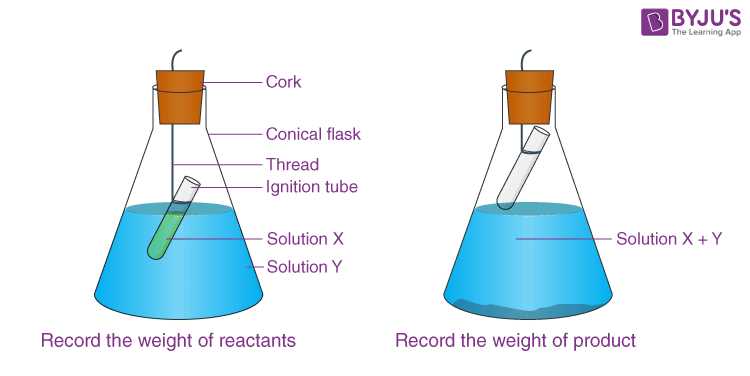
Rather than passively reading through material, actively engage with it by practicing problems and solving real-world applications. Use various methods, such as:
- Practice Problems: Regularly solving exercises helps reinforce theoretical knowledge and develops problem-solving skills.
- Flashcards: Create flashcards for formulas, key concepts, and important definitions to improve recall.
- Group Discussions: Collaborate with peers to explain difficult concepts. Teaching others is a powerful way to solidify your understanding.
By incorporating these active learning methods, you are more likely to retain information and apply it effectively in different scenarios.
What to Expect in Chemistry Exams
Exams in scientific disciplines often require a comprehensive understanding of concepts and the ability to apply them in various contexts. In this field, exams are designed to assess both theoretical knowledge and practical problem-solving skills. Preparing for these assessments involves a strategic approach, focusing on the key concepts, critical thinking, and the application of various methods to different scenarios.
Typically, these exams include multiple types of questions that test your ability to recall information, interpret data, and solve complex problems. The format may vary, but you can expect a combination of:
| Question Type | Description |
|---|---|
| Multiple Choice | Tests your knowledge of fundamental concepts and definitions, often requiring quick recall of facts. |
| Short Answer | Requires concise responses that demonstrate a deeper understanding of specific topics or methods. |
| Problem Solving | Involves applying principles and formulas to solve calculations or interpret results from experiments. |
| Essay Questions | Assesses your ability to explain complex ideas, theories, and their applications in detail. |
To succeed, focus on understanding the underlying principles rather than memorizing facts. Practice problem-solving techniques, review key topics, and ensure you are comfortable with both theoretical and practical aspects of the subject. Proper preparation is key to performing confidently on exam day.