
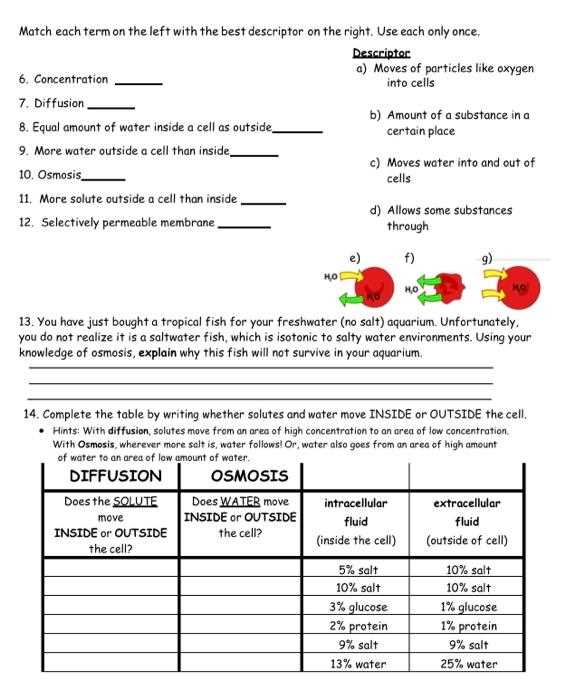
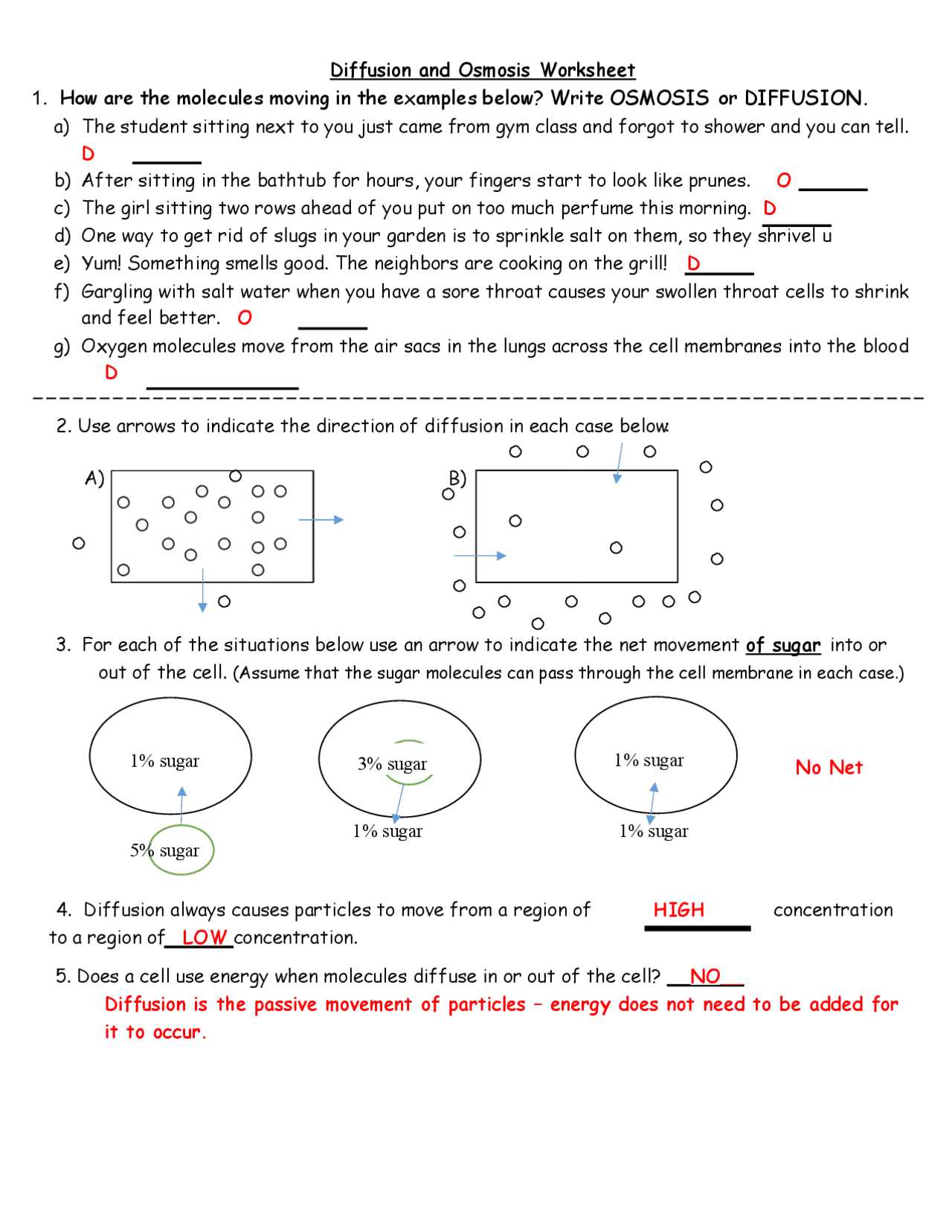
Understanding the movement of substances across cell membranes is a fundamental concept in biology. The process through which water and other molecules pass in and out of cells plays a crucial role in maintaining cellular functions. This section will provide insights into how these mechanisms operate in different environments, ensuring balance within the cell.
Through various exercises, students can explore the principles behind the movement of molecules, observe the effects of different conditions on cellular behavior, and deepen their comprehension of biological processes. This guide will help clarify key points and offer clear solutions to common challenges encountered when studying these processes.
Grasping these concepts is essential for anyone studying biology, as they form the basis for understanding more complex topics related to cell physiology and homeostasis. As you explore this material, you will gain a more detailed and practical understanding of how cells interact with their surroundings.
Osmosis Practice Activity Answer Key
This section provides solutions to the exercises designed to explore the movement of water and other molecules through cell membranes. By understanding the results and the reasoning behind them, students can better grasp the underlying principles of biological transport mechanisms.
The following points outline the essential takeaways from the exercise and offer explanations for each scenario presented. These solutions will help clarify any confusion and provide a step-by-step breakdown of the concepts involved:
- Cell behavior in different environments: Understand how cells react when exposed to varying concentrations of substances in their surroundings.
- Impact of solution types: Learn about the effects of hypertonic, hypotonic, and isotonic solutions on the movement of water across the cell membrane.
- Diffusion and selective permeability: Recognize how selective permeability of the membrane affects the movement of molecules.
- Observing equilibrium: Explore the concept of equilibrium in cellular systems and its significance for maintaining internal balance.
To reinforce the concepts, follow the outlined steps and check your findings against the provided explanations. This will ensure a deeper understanding of how substances move in and out of cells under different conditions.
- Review the experimental setup and conditions provided in each case.
- Compare your results with the expected outcomes based on cellular transport principles.
- Identify any discrepancies and consider the factors that might have influenced the results.
With these insights, you will be better equipped to understand how cells regulate their internal environments and how various conditions influence their behavior. This knowledge is fundamental in studying more complex biological processes.
Understanding Osmosis in Biological Systems
In biological systems, the movement of water and other essential molecules through cell membranes is crucial for maintaining life. This movement plays a key role in regulating the internal environment of cells, allowing them to function properly. Understanding how these substances travel across membranes is vital for grasping many fundamental processes in biology.
Cells are constantly interacting with their environment, adjusting to changes in surrounding concentrations of solutes. The ability of the cell membrane to selectively allow certain molecules to pass while blocking others is a critical feature that helps regulate cellular functions. This selective movement ensures that cells can maintain homeostasis, a state of balanced internal conditions.
To better understand how this process works, consider the following table summarizing different types of environments and their impact on cellular functions:
| Solution Type | Effect on Cell | Examples |
|---|---|---|
| Hypertonic | Cell loses water, shrinks | Saltwater solutions |
| Hypotonic | Cell gains water, may burst | Freshwater solutions |
| Isotonic | No net water movement, balanced | Balanced saline solutions |
This simple overview highlights how environmental factors influence the movement of molecules into and out of cells. Each type of solution can have different consequences for cell structure and function, which is why understanding these concepts is essential for studying cellular biology. By examining these mechanisms in detail, we gain insight into how cells maintain their delicate balance and adapt to changing conditions.
What Is Osmosis and How It Works
At its core, the process that allows substances to move through cell membranes is essential for the survival of living organisms. This movement involves the flow of water and other molecules from areas of higher concentration to areas of lower concentration. Understanding this process helps explain how cells maintain balance and respond to external conditions.
Cells are surrounded by membranes that act as barriers, allowing only certain molecules to pass through. The movement of water, in particular, plays a vital role in regulating cell function and supporting the cell’s internal environment. This process is driven by differences in concentration between the inside of the cell and its surroundings, aiming to achieve equilibrium.
The table below summarizes the main concepts involved in the process and highlights the different conditions that affect the movement of molecules:
| Process | Definition | Key Factor |
|---|---|---|
| Diffusion | Movement of molecules from high to low concentration | Concentration gradient |
| Selective Permeability | Ability of membranes to allow only certain molecules to pass | Membrane properties |
| Equilibrium | State where molecules are evenly distributed on both sides of the membrane | Concentration balance |
In biological systems, this movement of water helps to balance the pressure inside and outside the cell, ensuring proper cellular function. By understanding how molecules move across cell membranes, we gain insight into vital processes such as nutrient absorption, waste removal, and overall cellular health.
Key Concepts of Osmosis in Cells
In order to understand how cells interact with their environment, it is essential to explore the mechanisms that govern the movement of water and solutes across the cell membrane. These processes are fundamental to cellular function, as they help regulate the internal conditions of the cell, allowing it to maintain balance and respond to external changes.
Membrane Selectivity and Permeability
The cell membrane plays a crucial role in controlling what enters and exits the cell. It is selectively permeable, meaning it allows only certain molecules to pass while blocking others. This selective permeability is vital for maintaining homeostasis and ensuring that the cell’s internal environment stays within the optimal range for its activities.
Concentration Gradients and Water Movement
Water moves through the cell membrane according to concentration gradients, where it flows from regions of lower solute concentration to areas of higher solute concentration. This movement helps balance the concentration of substances inside and outside the cell, maintaining a stable internal environment. The direction of water movement is influenced by factors like solute concentration and pressure, which determine how water distributes itself across the membrane.
Understanding these concepts is essential for grasping how cells manage their interactions with the surrounding environment. These processes support vital cellular functions, from nutrient absorption to waste removal, ensuring the cell’s survival and proper function in a variety of conditions.
Types of Osmosis: Hypertonic Hypotonic Isotonic

When studying how substances move across cell membranes, it’s important to understand the different environments in which cells operate. The surrounding solutions can have various effects on the movement of water and other molecules. These environments are categorized into three main types based on their solute concentrations relative to the cell’s internal conditions.
The following list outlines the key differences between these three types of environments:
- Hypertonic – A solution with a higher concentration of solutes compared to the inside of the cell. Water moves out of the cell, causing it to shrink.
- Hypotonic – A solution with a lower concentration of solutes than the cell’s interior. Water moves into the cell, leading to expansion and potential bursting.
- Isotonic – A solution with an equal concentration of solutes inside and outside the cell. There is no net movement of water, maintaining the cell’s size and shape.
These environments play a significant role in how cells manage their internal pressure and maintain stability. Understanding how each solution affects the cell is crucial for studying cellular behavior under different conditions, and for applications in fields such as medicine, agriculture, and biotechnology.
Diffusion vs Osmosis Explained
Both diffusion and the movement of water across membranes are essential processes for maintaining cellular function. While they share some similarities, they differ in the way molecules move and the mechanisms involved. Understanding these two processes helps clarify how substances are transported in and out of cells, playing a key role in various biological functions.
Diffusion refers to the movement of molecules from an area of higher concentration to an area of lower concentration. This process continues until equilibrium is reached, meaning the concentration of molecules becomes equal throughout the space. It occurs with various types of molecules, such as gases, liquids, and solutes, and does not require energy.
In contrast, the movement of water through semi-permeable membranes is governed by different rules. Water moves in response to concentration gradients of solutes, typically from areas of low solute concentration to areas of high solute concentration. This process ensures that cells maintain their internal balance, though it involves the specific properties of the cell membrane that selectively allow certain substances to pass through.
While both diffusion and the movement of water are driven by concentration gradients, the key difference lies in the substances involved and the type of membrane required for their movement. Diffusion can occur in any solution or medium, while the movement of water requires a membrane that can control which molecules pass through. Both processes are vital for maintaining cellular homeostasis and ensuring the proper functioning of biological systems.
Importance of Osmosis in Plant Cells
Water movement plays a vital role in maintaining the structure and function of plant cells. This process helps regulate various physiological activities, such as nutrient uptake and waste removal, by ensuring that cells retain the necessary balance of water and solutes. For plants, this is especially crucial for their survival and growth in different environmental conditions.
In plant cells, the movement of water through the cell membrane is essential for maintaining turgor pressure, which keeps the cells firm and supports the plant’s overall rigidity. Without this pressure, plants would wilt and be unable to support their structures properly. The process helps the plant maintain a stable internal environment, allowing it to grow and thrive.
Key Benefits of Water Movement in Plant Cells:
- Nutrient Transport: Water helps dissolve minerals and nutrients, allowing them to travel through the plant’s vascular system and reach the necessary cells.
- Cell Expansion: The uptake of water into cells allows for their expansion, which is crucial for plant growth.
- Regulating Turgidity: Water maintains the pressure within cells, providing structural support and preventing wilting.
Overall, understanding how water moves in and out of plant cells helps explain many biological functions, from growth to the ability to withstand environmental stress.
Role of Osmosis in Animal Cells
The movement of water across cell membranes is essential for maintaining the internal balance of animal cells. This process helps regulate the cell’s environment, ensuring that nutrients are absorbed and waste is removed efficiently. It is also crucial for maintaining the cell’s shape and volume, which are vital for proper cellular function.
Maintaining Cell Volume and Pressure
In animal cells, water moves in and out through the cell membrane, driven by differences in solute concentration between the cell’s interior and its external environment. This movement helps control the volume of the cell, preventing it from swelling excessively or shrinking too much. Maintaining a stable volume is essential for cellular processes such as protein synthesis, energy production, and overall homeostasis.
Regulation of Internal Environment
Water movement also plays a significant role in the regulation of the internal environment, such as ion concentrations and pH levels. This process helps cells adapt to changes in the external environment, ensuring they maintain a stable internal state for optimal functioning. For example, cells in the kidneys rely on this process to filter waste from the bloodstream while preserving important substances like salts and water.
Key Functions of Water Movement in Animal Cells:
- Fluid Balance: Ensures that the cell maintains proper hydration and prevents dehydration or overhydration.
- Waste Removal: Helps flush waste products out of the cell, maintaining internal cleanliness.
- Maintaining Shape: Supports cell structure, preventing collapse or bursting under varying external conditions.
In essence, the movement of water within animal cells is vital for maintaining overall cellular health, stability, and adaptability to changing environments.
Steps to Solve Osmosis Practice Activity
To successfully understand and solve problems related to the movement of water and solutes across membranes, it is important to follow a systematic approach. This process helps clarify how substances interact within different environments, ensuring that each step of the problem is addressed effectively. Below are the general steps you can take to tackle such problems accurately.
First, it is essential to identify the key components involved in the problem, including the solute concentration inside and outside the cell, as well as the type of solution surrounding the cell. Knowing this will guide you in predicting the direction of water movement and understanding the effects on the cell.
Next, consider the behavior of water molecules based on the concentration gradients, determining whether water will enter, exit, or remain balanced within the cell. The goal is to predict what will happen to the cell in response to different solute concentrations.
Afterwards, apply any necessary equations or principles that relate to concentration differences and water movement. This might include understanding how pressure, concentration, and membrane permeability interact to influence the movement of water.
Finally, analyze the results of your calculations and observations to draw conclusions about the outcomes in the system. Reflecting on these results will help you better understand how different environments impact cells and provide insight into the broader implications of these biological processes.
Common Mistakes in Osmosis Activities
When studying the movement of substances across membranes, several common errors can arise, which may lead to misunderstanding the underlying principles. These mistakes can occur at different stages of the process, from misinterpreting concentrations to overlooking key factors that influence the movement of water and solutes. Below are some typical pitfalls to be aware of and avoid in these types of exercises.
- Misunderstanding Concentration Gradients: One of the most common mistakes is confusing the direction of water movement. It is crucial to remember that water moves from areas of low solute concentration to areas of high solute concentration, not the other way around.
- Overlooking the Role of the Membrane: Some students may forget that the permeability of the membrane plays a key role in determining which substances can pass through. A semi-permeable membrane only allows specific molecules to move, affecting the overall process.
- Failing to Consider Solution Types: Not recognizing the type of solution surrounding the cell (hypertonic, hypotonic, isotonic) can lead to incorrect predictions about the cell’s behavior. Each solution type has a distinct effect on the cell’s water balance.
- Ignoring Environmental Factors: Temperature and pressure can influence the rate at which substances move. Neglecting these factors can skew results and lead to inaccurate conclusions.
- Assuming Instant Results: The movement of water may take time, and assuming immediate results can lead to incorrect interpretations. It’s important to consider that these processes occur gradually over a period of time.
By being aware of these common mistakes, one can approach these exercises more accurately and avoid errors that hinder understanding of the processes involved in cellular functions.
Understanding Osmotic Pressure and Its Effects

Osmotic pressure is a critical concept in the movement of fluids across membranes. It refers to the force required to prevent the flow of water from one side of a selectively permeable membrane to the other, driven by differences in solute concentration. This pressure plays a vital role in maintaining the stability of cells and tissues, influencing the balance of fluids within an organism.
When two solutions with different solute concentrations are separated by a semi-permeable membrane, the solution with a higher concentration of solutes will attract water, creating a pressure that can have various effects on the cells or organisms involved. This pressure is essential for processes like nutrient absorption, waste removal, and the maintenance of cell shape and size.
Key Effects of Osmotic Pressure:
- Cell Turgidity: In plant cells, osmotic pressure helps maintain turgidity, providing structural support and preventing wilting.
- Fluid Regulation: In animal cells, osmotic pressure ensures the regulation of internal fluids, maintaining homeostasis and preventing dehydration or swelling.
- Transport Efficiency: Osmotic pressure aids in the efficient transport of essential substances like nutrients and waste products across cell membranes.
Understanding osmotic pressure and its effects is crucial for grasping how living organisms manage internal fluid balance and adapt to different environmental conditions.
Factors Affecting Osmosis in Cells

The movement of water and solutes across cell membranes is influenced by a variety of factors that determine the direction and rate at which these substances travel. These factors can significantly impact cellular function and overall organismal health, and understanding them is essential for comprehending how cells maintain equilibrium in different environments.
Concentration Gradient
The difference in solute concentration between the inside of the cell and the surrounding solution is one of the most important factors influencing fluid movement. A steeper concentration gradient (a larger difference in solute concentrations) leads to faster water movement towards the area with higher solute concentration. This can result in cells either swelling or shrinking depending on the direction of the water flow.
Temperature
Temperature affects the kinetic energy of molecules, which in turn impacts the rate of movement. Higher temperatures typically increase the rate at which water molecules move across the membrane, speeding up the process. Conversely, cooler temperatures slow down this movement, which can affect cellular processes that depend on the efficient transport of water and nutrients.
Additional Factors:
- Membrane Permeability: The ability of the cell membrane to allow specific molecules to pass through can alter how effectively water and solutes move. Membranes that are more permeable to water allow faster flow.
- Pressure: External pressure applied to the cell can also influence the rate of movement, either assisting or inhibiting the flow of water through the membrane.
By understanding these factors, one can better predict and explain the effects of different environmental conditions on cells and the movement of substances within them.
How Osmosis Affects Water Movement
The movement of water across cell membranes is driven by differences in solute concentrations on either side of the membrane. This process plays a crucial role in regulating the internal environment of cells, ensuring proper function and stability. When water moves from one area to another, it typically flows towards the region with a higher concentration of solutes, aiming to balance the concentrations between the two sides.
The movement of water can lead to various changes in the cell. For example, when water flows into a cell, it may swell, potentially causing the cell to burst if the pressure becomes too high. On the other hand, if water moves out of the cell, the cell may shrink or become dehydrated. The ability of cells to manage this flow of water is critical for maintaining their structure and function, especially in environments with fluctuating water levels.
| Type of Movement | Effect on the Cell | Example Scenario |
|---|---|---|
| Water Influx | Cell Swelling | Plant roots absorbing water from the soil |
| Water Efflux | Cell Shrinkage | Red blood cells in a saline solution |
| Equilibrium | Stable Cell Volume | Cells in isotonic environments |
Key Factors: The movement of water depends on several factors, including the concentration gradient, temperature, and the permeability of the cell membrane. Understanding these factors helps explain how cells adapt to different environments and maintain homeostasis.
Analyzing Osmosis Experiment Results
When conducting experiments involving the movement of water across membranes, the analysis of results is crucial to understanding how various factors influence this process. The data collected from such experiments can provide valuable insights into how solutes and solvents behave in different environments. Analyzing these results requires careful consideration of how variables such as concentration gradients, temperature, and membrane permeability affect the movement of water in and out of cells or artificial environments.
To interpret the results accurately, one must focus on several key observations. These include changes in the volume or mass of the cells or solutions over time, which can indicate whether water is entering or leaving the sample. The direction of water movement is directly tied to differences in solute concentrations, and tracking this movement helps to validate theoretical predictions.
Common methods for analyzing results include comparing the initial and final measurements of mass or volume, and calculating the rate of movement under different conditions. Additionally, graphical representations such as concentration vs. time plots or comparison tables can help visualize trends and make the data more comprehensible.
Key Points to Consider:
- Change in Mass/Volume: A noticeable increase in mass or volume often suggests water intake, while a decrease typically indicates water loss.
- Rate of Movement: The speed at which changes occur can reveal insights into the permeability of the membrane and the concentration differences between solutions.
- Consistency of Results: Consistency across multiple trials helps confirm the reliability of the experiment and its outcomes.
By understanding these factors and applying critical thinking, one can draw meaningful conclusions from experimental data, helping to explain the broader principles of water transport in living organisms and laboratory setups.
Using Osmosis to Teach Cell Functions
Understanding the movement of water and solutes across cell membranes is essential for grasping how cells perform their functions. This process plays a critical role in maintaining homeostasis within cells, affecting everything from nutrient absorption to waste removal. By teaching this concept through hands-on experiments and simulations, students can better appreciate the intricate balance that sustains life at the cellular level.
Interactive Learning Approaches
Using practical demonstrations and experiments allows students to observe how changes in the environment, such as the concentration of solutes, influence cellular processes. These experiments can be done with simple materials, such as plant cells or artificial membranes, to show how water moves in response to different conditions.
- Hands-On Simulations: Simulating various environments can help students visualize how cells interact with their surroundings, making abstract concepts more concrete.
- Real-World Examples: Relating the topic to real-life biological systems, like how kidneys filter waste or how plant roots absorb water, makes the lesson more relatable.
Key Concepts for Teaching
To effectively use this concept for teaching, it is important to focus on the following key ideas:
- Concentration Gradients: Understanding how differences in concentration drive the movement of substances across membranes is a fundamental principle of cellular function.
- Membrane Permeability: Discussing the selective nature of cell membranes helps students understand why certain substances can pass through while others cannot.
- Impact on Cell Health: Demonstrating how improper balance of water and solutes can lead to problems like dehydration or swelling helps students connect these processes to health and disease.
By incorporating this topic into lessons, educators can not only teach important biological concepts but also encourage critical thinking about how the smallest changes in cellular conditions can lead to significant effects on overall organism health.
How Osmosis Relates to Homeostasis
The movement of water and solutes across cell membranes is integral to maintaining the stable internal conditions necessary for life. Cells rely on this process to regulate their internal environment, ensuring that factors such as temperature, pH, and the concentration of nutrients and waste products remain within a narrow range. This equilibrium is crucial for the proper functioning of cells, tissues, and organs in all living organisms.
Maintaining Internal Balance
At the cellular level, the ability to control water movement helps organisms maintain homeostasis. Cells constantly adjust the flow of water in and out of their structures to balance the concentrations of different substances inside and outside the cell. This dynamic regulation ensures that the cell environment remains stable, allowing it to perform its functions effectively.
- Fluid Regulation: The movement of water plays a central role in maintaining fluid balance within cells, which is essential for nutrient transport and waste removal.
- Cell Volume Control: By regulating the amount of water entering or leaving the cell, organisms prevent the cell from becoming too swollen or dehydrated, which could disrupt its functions.
- Ion Concentration Management: Cells rely on water movement to help manage the concentration of ions, which is critical for processes like nerve signal transmission and muscle contraction.
Homeostatic Mechanisms in Action
Several homeostatic mechanisms depend on the movement of water across membranes, including:
- Kidney Function: The kidneys use water movement to filter out waste products while conserving water and essential ions, maintaining balance in the body.
- Plant Water Regulation: In plants, water movement through roots, stems, and leaves helps maintain turgor pressure and regulate nutrient absorption.
- Thermoregulation: In many organisms, water movement helps regulate body temperature by promoting the evaporation of excess heat.
Ultimately, the ability to regulate water and solute movement ensures that cells can function properly and adapt to changing environmental conditions, keeping the entire organism in balance. This process is central to homeostasis and is vital for the survival of all living organisms.
Practical Applications of Osmosis Knowledge
The fundamental principles of water movement across membranes have wide-ranging applications in both biological and technological fields. Understanding how substances move between different environments is critical for a variety of industries, from medicine to agriculture. By applying this knowledge, we can improve processes such as water purification, food preservation, and even medical treatments.
Medical and Healthcare Applications

One of the most important uses of this concept in healthcare is the development of treatments that rely on the regulation of fluids and solutes within the body. Here are a few key areas where this knowledge is applied:
- Dialysis: This process mimics the function of kidneys by removing waste products from the blood and balancing electrolyte levels through selective diffusion.
- Intravenous (IV) Fluids: Understanding the balance of solutes and water allows for the correct formulation of IV solutions to maintain blood volume and electrolytes in patients.
- Drug Delivery: Osmotic pressure is used to design controlled-release drug delivery systems, ensuring the sustained release of medication over time.
Agricultural and Industrial Applications
In agriculture and industry, understanding how substances move across membranes can improve crop irrigation techniques, food preservation methods, and even industrial filtration systems:
- Crop Irrigation: Understanding how water moves through plant roots allows farmers to better manage water usage and prevent over-irrigation or dehydration.
- Food Preservation: The application of principles of water movement is used in processes like salting, pickling, and drying to draw out moisture and inhibit bacterial growth.
- Water Filtration: In water purification, membranes can filter out unwanted particles through selective permeability, providing clean water for consumption.
Table: Examples of Osmotic Principles in Different Fields
| Field | Application | Benefit |
|---|---|---|
| Healthcare | Dialysis | Removes waste and balances electrolytes in the blood |
| Healthcare | IV Fluids | Maintains blood volume and electrolyte balance |
| Agriculture | Crop Irrigation | Optimizes water usage and prevents dehydration |
| Industry | Food Preservation | Inhibits microbial growth by removing moisture |
| Industry | Water Filtration | Purifies water by removing contaminants |
In conclusion, knowledge of how water and solutes move across membranes has profound implications, ranging from improving medical treatments to optimizing agricultural practices. This understanding drives innovation and efficiency in several critical industries, making it an invaluable area of study.
Tips for Mastering Osmosis Concepts

To fully understand how water and solutes move across biological membranes, it’s important to grasp the fundamental concepts that govern this process. With a strong foundation, you’ll be able to apply these principles to various scenarios, from cell function to real-world applications. Here are some tips to help you master these crucial ideas:
1. Build a Strong Foundation
Before diving into complex problems or applications, ensure you have a solid grasp of the basic principles. Understanding terms like diffusion, concentration gradients, and selective permeability will set the stage for more advanced concepts.
- Review Basic Terminology: Get familiar with key concepts like hypertonic, hypotonic, and isotonic solutions, as they are fundamental to understanding fluid movement.
- Understand the Role of Membranes: Membranes play a critical role in regulating what enters and exits a cell. Focus on how selective permeability influences this process.
2. Visualize the Process
Sometimes abstract concepts are easier to understand when you can see them in action. Try visualizing how molecules move from areas of higher concentration to lower concentration. This can help you better understand the underlying principles at work.
- Use Diagrams: Sketching out the movement of water molecules across membranes can clarify how solute concentration affects flow.
- Simulations and Models: Interactive tools and simulations can help demonstrate real-time changes that occur during fluid movement, reinforcing key concepts.
3. Apply Knowledge to Real-Life Examples
Relating theoretical knowledge to real-world applications can solidify your understanding. Here are some examples where you can see these principles in action:
- Plant Watering: Understand how water movement in plants follows the same principles to maintain hydration and nutrient uptake.
- Medical Applications: Study how treatments like dialysis or IV fluids rely on these principles to balance water and solutes in the human body.
- Food Preservation: Learn how methods like salting or pickling prevent bacterial growth by drawing water out of cells through selective permeability.
4. Practice Problem Solving
The best way to truly master any scientific concept is through practice. Work on a variety of problems that challenge you to apply your knowledge of how molecules move through membranes.
- Solve Conceptual Questions: These will help reinforce your understanding of the process and force you to think critically about how different factors influence fluid movement.
- Work on Calculations: Practice solving problems related to concentration gradients, diffusion rates, and equilibrium to strengthen your analytical skills.
5. Collaborate and Discuss
Sometimes discussing concepts with peers or instructors can open up new perspectives and help clarify confusing topics. Collaboration can be especially useful when tackling complex scenarios or questions.
- Join Study Groups: Working with others provides a chance to learn from different viewpoints and reinforce your understanding of key concepts.
- Ask Questions: Don’t hesitate to seek clarification on topics that are unclear. Engaging with experts or peers can lead to deeper insights.
By following these tips and remaining consistent with your learning, you’ll be well on your way to mastering the principles that govern fluid movement and understanding their significance in biological systems.