
In the field of science, the exploration of the nature of matter has led to the development of various models and frameworks that attempt to explain its structure and behavior. These representations have evolved over time, shaped by groundbreaking discoveries and new technologies. Each stage in this intellectual journey has provided a deeper understanding of how substances interact at the most basic levels.
As we delve into the historical progression of these concepts, we observe the significant contributions of renowned scientists who have shaped the way we perceive the building blocks of the universe. From early theories to modern-day interpretations, the shift in our understanding is both fascinating and essential for advancing scientific thought.
By studying these key perspectives, learners gain valuable insights into the principles that govern the behavior of matter. These frameworks not only provide answers to longstanding questions but also open the door to new possibilities for exploration in the ever-evolving landscape of science.
Webquest Atomic Theories and Models Answers
This section provides an in-depth exploration of the key concepts that describe the structure of matter. It outlines various scientific perspectives that have evolved over centuries, giving a clear understanding of how these frameworks have shaped our knowledge of the physical world. By reviewing these essential ideas, learners can grasp the fundamental principles behind the behavior and interactions of particles.
Several significant stages in the development of these ideas have occurred, each one introducing new insights and refining previous concepts. These stages not only help to explain basic phenomena but also contribute to technological advancements and scientific breakthroughs.
- First, it is essential to grasp the early models that laid the foundation for later developments.
- Next, understanding how later contributions refined these initial concepts is key to grasping the progress made in scientific thought.
- Additionally, modern perspectives often combine elements from earlier theories to form more comprehensive frameworks.
Each stage in this progression has its own set of interpretations and methods of explaining phenomena. By exploring these perspectives, students can develop a deeper understanding of the principles that guide current scientific knowledge.
- Study the basic principles behind the earliest conceptualizations of matter.
- Examine how scientists built upon these initial ideas with more sophisticated approaches.
- Understand the role of experimental evidence in shaping our current understanding of physical substances.
Through this examination, one can appreciate the collaborative nature of scientific discovery, where each contribution builds on the work of others. These frameworks are not static; they continue to evolve as new information and technology allow us to observe and manipulate the smallest components of matter with unprecedented precision.
Understanding Atomic Theory Basics

At the core of scientific exploration is the concept of matter’s structure, which has been the subject of investigation for centuries. The pursuit of understanding how substances are built from the smallest components has led to the development of foundational ideas. These concepts serve as the groundwork for exploring the nature of physical reality, helping us understand everything from the simplest materials to the most complex phenomena in the universe.
Early Foundations of Matter Structure
In the early stages of scientific inquiry, thinkers proposed ideas about the composition of matter, suggesting that it is made up of indivisible particles. These initial propositions were based on observation and reasoning, laying the foundation for more sophisticated investigations in the future. Over time, these ideas evolved as new evidence was discovered and more advanced tools became available for testing hypotheses.
Key Contributors to the Concept of Matter
Throughout history, many prominent scientists have made pivotal contributions to refining our understanding of matter’s fundamental units. Their work collectively shaped the modern perspective on how substances are organized. These contributions include important breakthroughs in identifying the building blocks of matter, as well as recognizing the behaviors and interactions between them. By studying their work, we gain insight into the progression of ideas that continue to influence scientific thought today.
Key Contributors to Atomic Models
The development of our current understanding of the structure of matter has been significantly influenced by several brilliant minds throughout history. These individuals, through their experiments and theories, have helped shape the way we perceive the basic building blocks of the universe. Each contribution built upon the last, gradually refining our knowledge and leading to the more detailed frameworks we use today.
John Dalton is often credited with introducing one of the first modern interpretations of matter’s composition in the early 19th century. His work laid the foundation for understanding how substances are made of indivisible particles, a concept that greatly influenced future studies.
J.J. Thomson, in the late 19th century, revolutionized the way we think about matter by discovering the existence of electrons. His plum pudding model suggested that negative particles were embedded in a positively charged “soup,” reshaping our view of the atom’s internal structure.
Ernest Rutherford made a groundbreaking discovery in the early 20th century, showing that atoms have a dense, positively charged nucleus. His gold foil experiment led to the realization that the atom is mostly empty space, which was a major step forward in understanding its internal structure.
Niels Bohr further refined the concept with his planetary model, proposing that electrons orbit the nucleus in discrete energy levels. This model was crucial in explaining the behavior of atoms, particularly how they absorb and emit light.
These scientists, among many others, played pivotal roles in forming the ideas that have guided countless discoveries in science. Their work continues to influence our understanding of the universe at its most fundamental level.
Importance of Atomic Theory in Science
The study of the fundamental components of matter has been crucial to the advancement of science. Understanding how the smallest particles interact and combine provides the foundation for countless discoveries across various fields. This knowledge not only helps explain natural phenomena but also drives innovation in technology, medicine, and energy, transforming our daily lives.
Scientific Progress relies on models that accurately depict how matter behaves. By exploring these models, researchers are able to predict the properties of substances, guiding experimentation and technological development. For instance, the understanding of electron behavior has led to the creation of devices like semiconductors, which are essential for modern electronics.
Practical Applications of these frameworks have also reshaped industries such as medicine, where an understanding of molecular interactions has been pivotal in drug development and diagnostics. The ability to manipulate matter at a microscopic level opens up new possibilities in treatments and therapies that were once unimaginable.
In addition, the role of these ideas extends beyond laboratory experiments. They influence our understanding of energy sources, environmental science, and the behavior of materials, making them fundamental not just to science but to everyday life.
Historical Development of Atomic Concepts
The journey of understanding the nature of matter spans thousands of years, beginning with early philosophical musings and evolving into the precise scientific frameworks used today. This intellectual progression has been shaped by key discoveries and groundbreaking experiments that gradually revealed the fundamental structure of substances and their behavior. As new tools and methods emerged, scientists were able to refine their ideas, leading to a deeper comprehension of the physical world.
Early ideas about the composition of matter were rooted in philosophy. Thinkers like Democritus proposed that everything is made up of indivisible particles, though these ideas were largely speculative and lacked empirical support. It wasn’t until the development of more sophisticated experimental techniques that these concepts could be tested and verified.
- John Dalton (early 1800s) laid the foundation of modern understanding with his proposition that matter is made up of small, indivisible particles called atoms, each with specific characteristics.
- J.J. Thomson (1897) discovered the electron, challenging previous views and suggesting that the atom could be divided into smaller components.
- Ernest Rutherford (1911) proposed that atoms have a small, dense nucleus at their center, fundamentally changing how we think about the atom’s internal structure.
As the 20th century progressed, ideas continued to evolve with the development of quantum mechanics. This new framework allowed for a better understanding of the behavior of particles at the subatomic level, providing explanations for phenomena that earlier models could not account for.
- In the early 20th century, Niels Bohr introduced the concept of energy levels in the atom, explaining how electrons move within specific orbits.
- Later, quantum mechanics expanded upon Bohr’s ideas, offering a probabilistic view of electron positions, which proved to be a more accurate representation of atomic behavior.
These milestones reflect the gradual shift from abstract ideas to a more precise, evidence-based understanding of matter. Each discovery has built upon the last, creating a comprehensive framework that continues to shape scientific research today.
Dalton’s Atomic Theory Explained
In the early 19th century, the work of John Dalton introduced a revolutionary way of understanding the composition of matter. His theory laid the groundwork for modern chemistry by providing a structured explanation of how substances are composed of indivisible particles. Dalton’s ideas were based on the premise that all matter is made up of tiny, fundamental particles, which he called “atoms,” each with its own unique properties.
Dalton’s model presented several key concepts that changed the way scientists thought about matter. He proposed that these particles could not be subdivided or destroyed in chemical reactions, a radical idea at the time. This concept helped explain why certain chemical reactions resulted in the same ratios of substances, no matter the amount involved. Dalton’s theory marked the beginning of a more systematic approach to chemical reactions and molecular interactions.
| Dalton’s Key Postulates | Explanation |
|---|---|
| 1. All matter is made of indivisible particles called atoms. | Atoms cannot be created, destroyed, or divided during chemical reactions. |
| 2. Atoms of the same element are identical in mass and properties. | Elements are made up of atoms that share the same physical and chemical characteristics. |
| 3. Compounds are formed when atoms of different elements combine in fixed ratios. | Substances are created through the combination of atoms from different elements in specific proportions. |
| 4. Chemical reactions involve the rearrangement of atoms. | Atoms are neither created nor destroyed in reactions; they simply rearrange to form new substances. |
Dalton’s work provided a logical explanation for the behavior of substances in chemical reactions, helping to transform chemistry from an observational science into one based on principles that could be tested and proven. Though later advancements in particle physics would refine and adjust Dalton’s ideas, his theory remains a cornerstone of modern scientific thought.
Thomson’s Model and Discoveries
In the late 19th century, the understanding of matter took a dramatic turn with the discoveries made by J.J. Thomson. His experiments led to groundbreaking findings that changed how scientists viewed the structure of substances. Thomson’s work provided the first clear evidence that atoms contain smaller, negatively charged particles. This discovery fundamentally altered the perception of the atom as an indivisible unit and opened the door to further exploration of its internal structure.
Thomson’s Experiment and the Electron
Thomson’s most significant contribution came in 1897 when he identified the electron using a cathode ray tube. He demonstrated that these rays were composed of negatively charged particles, which were much smaller than the atom itself. This discovery proved that atoms are not indivisible, as previously believed, but are made up of even smaller components.
Thomson’s findings led him to propose a new model of the atom, often referred to as the plum pudding model. In this model, he suggested that the atom was composed of a positively charged “soup” in which negatively charged electrons were embedded, much like raisins in a plum pudding. This was a revolutionary idea at the time, providing a new framework for thinking about atomic structure.
Key Discoveries and Their Impact
Thomson’s experiments not only identified the electron but also had a profound influence on future scientific developments. His work laid the foundation for the study of subatomic particles and atomic structure, setting the stage for later experiments that would refine and build upon his initial ideas.
| Thomson’s Discoveries | Impact on Atomic Understanding |
|---|---|
| Discovery of the Electron | Revealed that atoms contain smaller particles, challenging the idea of indivisible matter. |
| Plum Pudding Model | Introduced the concept of an atom with embedded electrons, laying the groundwork for later atomic theories. |
| Cathode Ray Experiment | Provided experimental evidence of subatomic particles, leading to a new understanding of atomic structure. |
While later experiments, such as Rutherford’s, would ultimately refine Thomson’s model, his discoveries were pivotal in expanding the understanding of matter at a subatomic level. Thomson’s work set the stage for future breakthroughs that would shape modern physics and chemistry.
Rutherford’s Contribution to Atomic Science
In the early 20th century, the understanding of matter underwent a significant transformation thanks to the groundbreaking work of Ernest Rutherford. His experiments and discoveries reshaped scientific perspectives on the structure of matter and the arrangement of particles within it. Rutherford’s insights built upon previous models and provided experimental evidence that led to the development of a more accurate representation of the atom.
Rutherford’s most famous contribution was his gold foil experiment, which provided the first evidence of a dense, positively charged nucleus at the center of the atom. Before this experiment, the prevailing theory was that the atom was a uniform mass with electrons scattered throughout, as described by J.J. Thomson. Rutherford’s findings, however, revealed a new structure that would become the foundation for modern atomic science.
The Gold Foil Experiment
In 1909, Rutherford and his colleagues conducted an experiment in which they fired alpha particles at a thin sheet of gold foil. Most of the particles passed through the foil, but some were deflected at large angles, while a few even bounced back. This unexpected result led Rutherford to propose that atoms consist of a small, dense nucleus that contains most of the atom’s mass, surrounded by a vast empty space in which electrons reside.
Key Insights from Rutherford’s Work
Rutherford’s contributions to the understanding of matter are numerous and far-reaching. His work not only led to the discovery of the nucleus but also laid the foundation for the later development of quantum mechanics and nuclear physics. Below are some of the key takeaways from his research:
- Discovery of the Nucleus: Rutherford’s experiment demonstrated that atoms have a small, dense nucleus at their center, challenging earlier models of the atom.
- Revised Atomic Structure: His findings suggested that the atom is mostly empty space, with a tiny, positively charged nucleus surrounded by orbiting electrons.
- Foundation for Nuclear Physics: Rutherford’s work opened the door to further research in nuclear science, leading to the discovery of the proton and later advancements in nuclear energy.
Rutherford’s work was a turning point in atomic theory, providing crucial insights that would influence future discoveries and scientific developments. His contributions to understanding the structure of matter helped shape the field of nuclear physics and contributed to a deeper understanding of the universe at its most fundamental level.
Bohr’s Model of the Atom
In the early 20th century, the understanding of the structure of matter took a significant leap forward thanks to the work of Niels Bohr. Bohr’s approach to the internal structure of the atom introduced a new way of thinking about how electrons move around the nucleus. His model was a breakthrough that combined classical physics with emerging concepts in quantum theory, offering a more accurate explanation of atomic behavior than previous ideas.
Bohr proposed that electrons orbit the nucleus in specific, quantized energy levels, rather than moving randomly or continuously. These orbits were associated with fixed amounts of energy, and electrons could jump between these levels by absorbing or emitting a quantum of energy. This explanation helped to clarify many observations that earlier models couldn’t account for, such as the emission spectra of atoms.
Key Features of Bohr’s Model
Bohr’s model was revolutionary because it explained several phenomena that previous models couldn’t, especially when it came to the interaction of atoms with light. Below are the key features of Bohr’s approach:
- Energy Levels: Electrons move in fixed orbits around the nucleus, each associated with a specific energy level. These levels are quantized, meaning that electrons can only exist in certain stable orbits.
- Electron Transitions: Electrons can jump from one energy level to another, but they must absorb or emit energy to do so. This explains the discrete lines observed in atomic emission spectra.
- Stability of Orbits: Electrons in these orbits do not radiate energy, which would otherwise cause them to spiral into the nucleus, as suggested by earlier models.
Impact of Bohr’s Work

Bohr’s model was groundbreaking because it provided a more detailed understanding of the atomic structure. His ideas helped to explain the line spectra of hydrogen and laid the groundwork for the development of quantum mechanics. While Bohr’s model has since been refined and expanded, particularly with the development of wave mechanics and the modern quantum theory, it remains a key milestone in the history of atomic science.
Quantum Theory and Atomic Behavior
The study of matter at its most fundamental level led to the development of quantum theory, which introduced a new understanding of how particles behave at microscopic scales. Unlike classical mechanics, which relied on predictable paths and behaviors, quantum mechanics revealed that particles, such as electrons, exhibit both particle-like and wave-like properties. This duality challenged previous assumptions and helped explain the strange behavior observed at the subatomic level.
One of the key aspects of quantum theory is that particles exist in a state of probability rather than certainty. Instead of being in one specific position at any given time, particles are described by a wave function that gives the probability of finding the particle in various locations. This idea, known as the uncertainty principle, was one of the key breakthroughs that explained atomic behavior that could not be accounted for by older theories.
Wave-Particle Duality
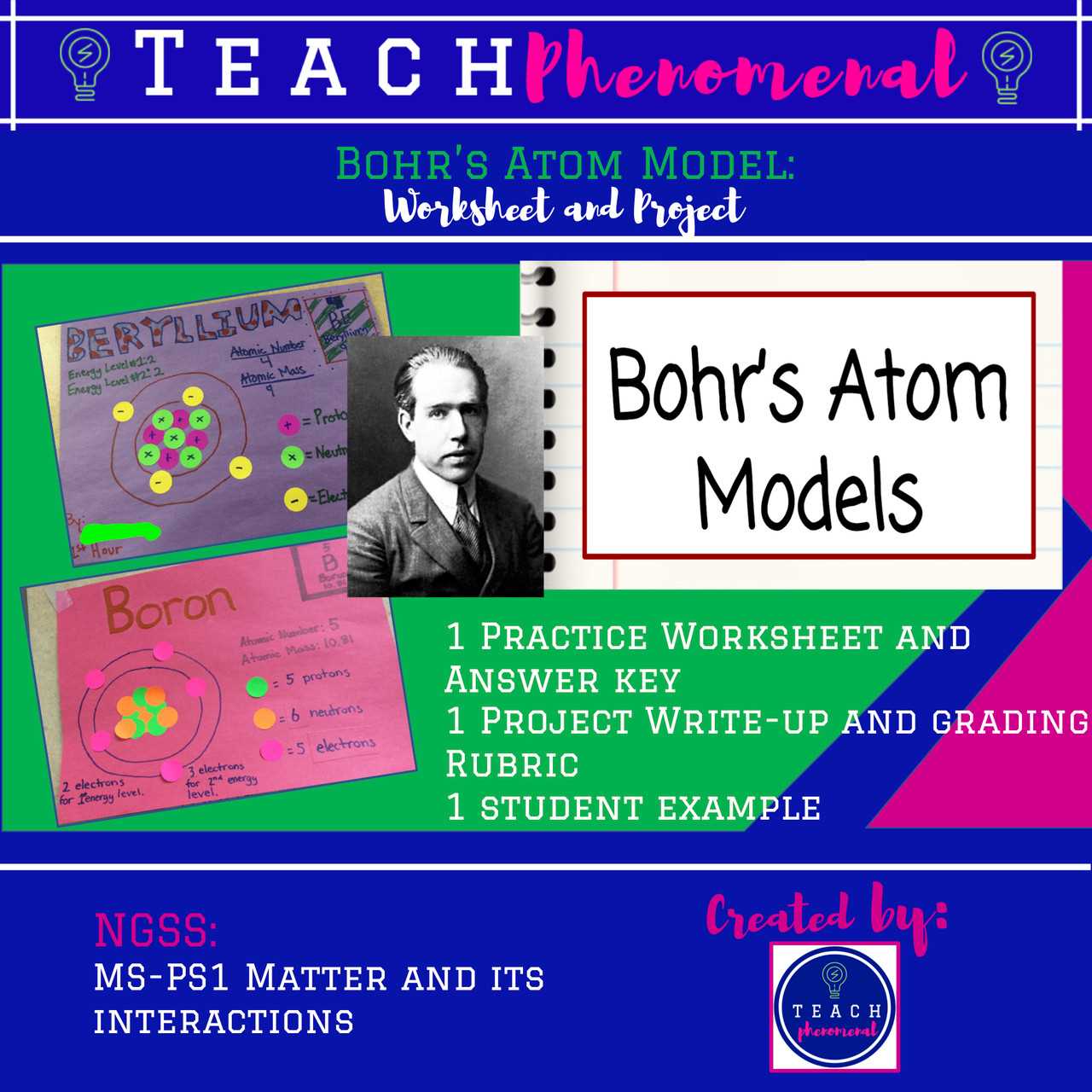
One of the core concepts in quantum mechanics is wave-particle duality. This principle suggests that particles like electrons do not have a single, fixed position or momentum but instead exhibit properties of both waves and particles. This was first observed in experiments such as the double-slit experiment, where electrons passing through two slits created an interference pattern, which is characteristic of waves. However, when observed individually, the electrons behaved like particles. This phenomenon has significant implications for understanding the behavior of electrons around a nucleus.
Quantum States and Energy Levels
In quantum mechanics, particles like electrons do not orbit the nucleus in fixed paths, as once thought. Instead, they occupy quantized energy levels, with each level corresponding to a specific energy state. Electrons can move between these levels by absorbing or emitting discrete amounts of energy, a process that explains atomic spectra. The uncertainty principle also suggests that it is impossible to know both the position and momentum of a particle with complete accuracy, which contrasts with earlier models of fixed orbits and predictable motions.
| Quantum Concept | Explanation |
|---|---|
| Wave-Particle Duality | Particles like electrons exhibit both wave-like and particle-like behavior depending on the experiment. |
| Uncertainty Principle | It is impossible to know both the exact position and momentum of a particle at the same time. |
| Quantized Energy Levels | Electrons exist in discrete energy states, moving between levels by absorbing or emitting specific amounts of energy. |
The development of quantum theory revolutionized our understanding of matter, offering a more comprehensive explanation for the behavior of particles at the atomic and subatomic levels. While the theory can seem abstract and counterintuitive, it has provided the framework for much of modern technology and continues to influence advancements in fields such as chemistry, electronics, and materials science.
Electron Cloud Model Overview
The electron cloud model provides a more accurate and modern view of how particles move within an atom. Unlike earlier theories that depicted electrons as traveling in fixed orbits, this model describes the region around the nucleus where electrons are likely to be found. Rather than having well-defined paths, electrons exist in a “cloud” of probability, where their exact location at any given moment cannot be precisely determined. This concept helps explain the behaviors of electrons in more complex systems and is a key element in our understanding of chemical bonding and atomic interactions.
Probabilistic Nature of Electron Location
One of the core ideas of the electron cloud model is that we can’t pinpoint the exact position of an electron at any given time. Instead, the model uses a mathematical function, called a wave function, to describe the probability of finding an electron in a particular region around the nucleus. This is a fundamental departure from older models, where electrons were thought to follow predictable, circular orbits. In the electron cloud model, the cloud represents areas where the probability of locating an electron is high.
Electron Orbitals and Energy Levels
In this model, electrons are not confined to fixed orbits but occupy specific energy levels or shells around the nucleus. Within these energy levels, there are sublevels that are further divided into orbitals. Each orbital can hold a specific number of electrons, with each orbital shaped differently depending on the energy sublevel. These orbitals are often described in terms of their shapes, such as spherical (s), dumbbell-shaped (p), or more complex configurations (d and f orbitals). This organization helps explain the arrangement of electrons in various elements and their chemical properties.
The electron cloud model was a critical advancement over earlier models, such as the Bohr model, and has proven to be an essential tool in chemistry and quantum physics. By using probability rather than certainty to describe electron positions, it provides a more accurate representation of the behavior of electrons, especially in more complex atoms and molecules.
Comparing Early Atomic Theories
The development of scientific thought regarding the structure of matter evolved over centuries, with various scholars offering their interpretations based on the knowledge and tools available to them. Early models sought to explain the nature of matter, focusing on the smallest units that composed everything around us. As science advanced, these ideas were refined and challenged, leading to a deeper understanding of the components that make up the universe. This section will compare the key early concepts and how each contributed to the evolving picture of matter’s fundamental structure.
Democritus’ Idea of the Indivisible Unit
In ancient Greece, Democritus proposed that all matter is made up of tiny, indivisible particles called “atoms.” While his ideas were more philosophical than scientific, they laid the groundwork for future exploration of the nature of matter. Democritus believed that atoms were eternal, indestructible, and in constant motion. Although his theories were not widely accepted at the time, they influenced later scientists who would build upon these ideas with more empirical methods.
Dalton’s Proposal: A Scientific Approach
Nearly two thousand years after Democritus, John Dalton provided a more structured and scientifically grounded model. Dalton’s theory, formulated in the early 19th century, proposed that each element consists of its own type of atoms, which combine in fixed ratios to form compounds. This marked the first step toward a more modern understanding of matter’s composition. Dalton’s model also suggested that atoms of the same element are identical in mass and size, although this would later be disproven as atomic theory evolved.
- Democritus’ Theory: Atoms are indivisible, eternal, and always in motion.
- Dalton’s Theory: Matter is composed of atoms that combine in fixed ratios to form compounds.
Thomson’s Discovery of Subatomic Particles
Another major shift occurred when J.J. Thomson discovered the electron in 1897. Thomson’s model, known as the “plum pudding model,” suggested that atoms were made up of a positively charged “pudding” with negatively charged “plums” (electrons) embedded within. Although this model was later replaced by more accurate representations, it was crucial in identifying the existence of subatomic particles and challenging the idea that atoms were indivisible.
Rutherford’s Gold Foil Experiment
In the early 20th century, Ernest Rutherford’s gold foil experiment led to the discovery of the atomic nucleus. His findings demonstrated that atoms have a dense, positively charged center (the nucleus) and that most of the atom is empty space. This discovery fundamentally changed our understanding of the structure of atoms, moving away from earlier models that did not account for this dense central core.
- Thomson’s Discovery: Atoms are composed of subatomic particles, including electrons.
- Rutherford’s Contribution: Atoms have a central nucleus, with electrons surrounding it in empty space.
As science advanced, these early theories were modified and replaced by more accurate models. The progress from Democritus’ philosophical ideas to Dalton’s experimental approach, and later to the discoveries of Thomson and Rutherford, reflects the growing sophistication of atomic theory. Each new discovery helped clarify the true nature of matter, leading to the modern understanding of atomic structure that continues to be refined today.
How Atomic Models Evolved Over Time
The understanding of the smallest building blocks of matter has undergone significant changes throughout history. As scientific knowledge advanced and new discoveries were made, early conceptualizations were refined or replaced with more accurate representations. This evolution reflects the increasing sophistication of experimental techniques, as well as a deeper understanding of the structure of matter. Over time, different scientists proposed various explanations, each contributing to a more complete picture of the components that make up everything around us.
Early Concepts of Matter
In ancient times, thinkers like Democritus believed in the idea of indivisible particles that made up all substances. Although his philosophy lacked experimental evidence, it marked the beginning of the journey toward understanding matter’s fundamental components. His view of particles being eternal and unchanging set the stage for later ideas, but it wasn’t until the 19th century that the concept was revisited in a more scientific context.
- Democritus’ Philosophy: Proposed the existence of indivisible particles, known as “atoms”.
- Early Limitations: Lacked empirical data and experimental verification.
Dalton’s Experiment-Based Approach
In the early 1800s, John Dalton developed a more scientific theory based on experimental observations. Dalton suggested that all elements were made of identical, indivisible particles, which combined in fixed proportions to form compounds. His approach marked a significant shift, as it was based on measurable data rather than philosophical speculation. Although this model would later be refined, Dalton’s work laid the groundwork for future developments in the field of chemistry.
- Dalton’s Theory: Matter is composed of indivisible particles (atoms) that combine in fixed ratios to form compounds.
- Key Contribution: Emphasized experimentation and empirical evidence in the understanding of matter.
Thomson’s Discovery of Electrons
In 1897, J.J. Thomson’s experiments with cathode rays led to the discovery of the electron, a negatively charged particle. This finding challenged the idea that atoms were indivisible and opened the door to the concept of subatomic particles. Thomson proposed the “plum pudding model,” in which electrons were embedded in a positively charged matrix. Although this model was later replaced, it was a crucial step in advancing our understanding of atomic structure.
- Thomson’s Discovery: Electrons are negatively charged particles within atoms.
- Plum Pudding Model: Atoms consist of a positively charged “pudding” with negatively charged electrons scattered throughout.
Rutherford’s Revolutionary Findings
In 1911, Ernest Rutherford conducted the gold foil experiment, which revealed that most of an atom’s mass is concentrated in a small, dense core. This led to the discovery of the nucleus, a concept that overturned the plum pudding model. Rutherford’s findings demonstrated that atoms are mostly empty space, with a central nucleus surrounded by electrons. This was a groundbreaking moment in the development of atomic theory and provided a much clearer picture of the atom’s internal structure.
- Rutherford’s Experiment: Discovered the nucleus, showing that atoms are mostly empty space.
- Rutherford’s Model: Proposed a central nucleus surrounded by electrons.
Bohr’s Quantized Model
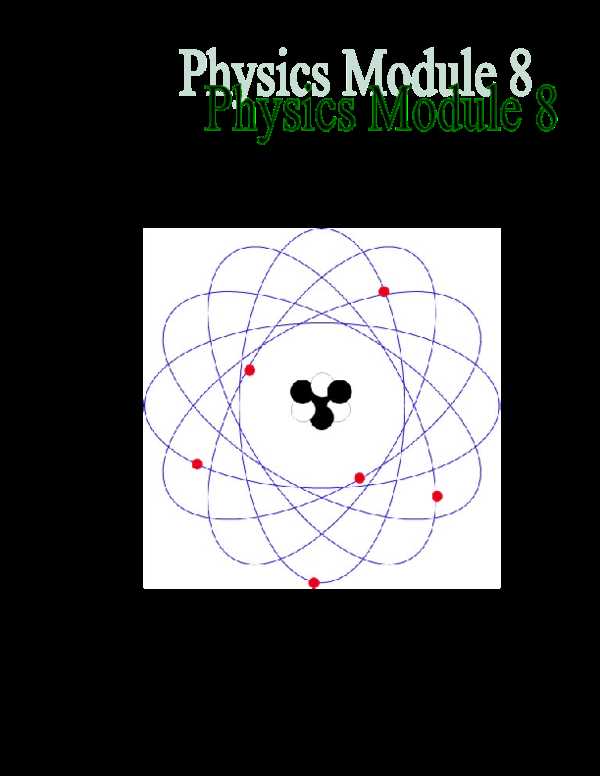
In 1913, Niels Bohr further refined Rutherford’s model by introducing the idea of quantized energy levels. Bohr proposed that electrons orbit the nucleus at fixed distances, with each orbit corresponding to a specific energy level. This model was able to explain the stability of atoms and the emission spectra observed in various elements. Though later refined by quantum mechanics, Bohr’s approach represented a major leap forward in the understanding of atomic structure.
- Bohr’s Contribution: Introduced the concept of quantized orbits for electrons.
- Energy Levels: Electrons occupy specific energy levels, each associated with a distinct orbit.
Quantum Mechanical Model
As science progressed further into the 20th century, the development of quantum mechanics brought even more profound changes to atomic theory. The quantum mechanical model, developed by Schrodinger, Heisenberg, and others, replaced the idea of fixed orbits with probabilistic electron clouds. This model recognizes that electrons do not have precise positions or velocities but exist in regions of probability around the nucleus, known as orbitals. This model provided a more accurate and comprehensive understanding of the behavior of subatomic particles.
- Quantum Mechanics: Replaced fixed orbits with probabilistic electron clouds.
- Electron Orbitals: Electrons exist in regions of probability, rather than specific orbits.
As scientific knowledge has continued to evolve, the model of the atom has become more complex and accurate, reflecting both theoretical advancements and experimental discoveries. From early philosophical musings to quantum mechanics, our understanding of the atom has transformed over time, continuing to shape modern science in profound ways.
Significance of Atomic Models in Education
Understanding the fundamental structure of matter is essential for students in many branches of science. Through conceptual frameworks and visual representations, complex ideas about the smallest building blocks of the universe are simplified for educational purposes. These explanations help students grasp difficult concepts, build a deeper understanding of the physical world, and foster critical thinking skills. By learning about the progression of ideas and the development of these frameworks, students can better appreciate the role of science in shaping our knowledge of the universe.
Enhancing Conceptual Understanding
One of the primary roles of these frameworks is to break down abstract concepts into more digestible parts. For students new to the subject, abstract concepts like the behavior of subatomic particles or the interaction between different components of matter can be difficult to grasp. Visualizations, models, and diagrams provide students with tangible representations of these otherwise invisible concepts, making learning both more accessible and engaging.
- Clarifying Complex Ideas: Providing clear visual tools to represent abstract ideas.
- Improving Retention: Helping students retain difficult concepts through engaging models and hands-on activities.
Fostering Curiosity and Critical Thinking
By studying the evolution of these frameworks, students also gain insight into how scientific thought has progressed over time. Learning about the mistakes, breakthroughs, and revisions that have taken place throughout history encourages curiosity and critical thinking. It provides students with a deeper appreciation for the scientific process, showing how each theory or framework represents an attempt to understand and explain the natural world. This encourages a mindset of inquiry, where students question, explore, and analyze their surroundings.
- Encouraging Inquiry: Stimulating curiosity and a desire to question the world around us.
- Developing Analytical Skills: Promoting critical thinking by encouraging students to understand the reasons behind revisions in these concepts.
Overall, understanding these frameworks not only provides a clear understanding of matter but also lays the groundwork for scientific literacy. As education continues to evolve, it is vital to integrate these models into teaching methods to ensure students are equipped with the tools needed to navigate the complexities of the scientific world.
Practical Applications of Atomic Theories
The understanding of the fundamental components of matter has led to significant advancements in various fields of science and technology. Insights into the structure of matter have been crucial for developing new materials, medical treatments, and energy sources. By applying concepts derived from the study of the smallest building blocks of matter, scientists and engineers have been able to create technologies that have far-reaching effects on our daily lives. These practical applications demonstrate the importance of scientific theories in shaping modern society.
One of the most notable areas where these concepts have been applied is in the development of electronics. The principles underlying the behavior of electrons in different materials are fundamental to the design of semiconductors and the creation of electronic devices. From smartphones to computers, the miniaturization of electronic components and the ability to control electron flow has revolutionized modern technology.
Another key area of application is in medicine, where these concepts have contributed to advancements in diagnostic tools and therapies. For instance, the use of radiation in cancer treatments, such as radiation therapy, relies on a deep understanding of subatomic particles and their interactions with cells. Similarly, imaging techniques like MRI and PET scans depend on principles that describe how particles behave in different environments.
In the energy sector, insights into matter’s structure have paved the way for nuclear energy, both in the form of nuclear power plants and in the use of radioactive isotopes for energy generation. Understanding how the nucleus of an atom behaves when subjected to various forces has allowed scientists to harness immense amounts of energy in controlled environments.
Overall, the practical applications of these theories extend far beyond the laboratory, influencing industries, healthcare, and technology. As science continues to progress, the knowledge of the smallest components of matter will remain a crucial driving force for future innovations.
Modern Interpretations of Atomic Models
The evolution of scientific thought has led to new perspectives on how the fundamental components of matter are structured and behave. As advancements in technology, particularly in particle physics and quantum mechanics, have deepened our understanding, the traditional views of matter have been refined and expanded. Today, scientists approach the behavior of matter with sophisticated models that incorporate both classical concepts and modern insights from quantum theory.
The Quantum Mechanical Model
One of the most significant advancements in the interpretation of matter is the development of the quantum mechanical framework. Unlike previous models, which depicted electrons in fixed orbits around the nucleus, this model treats electrons as existing in a cloud of probabilities rather than specific locations. The model relies on the principles of wave-particle duality, where electrons are not treated as particles with precise positions, but as wave-like entities that can exist in a range of possible locations around the nucleus. This approach allows for more accurate predictions about atomic behavior, especially at the subatomic level.
Emerging Theories and Technologies
As experimental techniques improve, new models are continually being proposed to explain atomic interactions in more detail. One such area of development is the study of quarks and gluons, which are considered fundamental components of protons and neutrons. These emerging theories aim to describe how subatomic particles interact at even smaller scales than previously imagined, offering insights into the structure of matter at a level that was once thought to be unreachable.
Additionally, advancements in computational modeling have allowed scientists to simulate the behavior of atoms in ways that were not possible in earlier years. By using powerful computers, researchers can now explore complex interactions between atoms, molecules, and materials, which leads to better understanding and innovative applications in various fields such as material science, energy storage, and nanotechnology.
These modern interpretations of matter provide a more complete and nuanced view of how substances interact at the smallest scales. They incorporate not only traditional ideas but also the latest research in quantum mechanics, particle physics, and computational chemistry, opening up new possibilities for technological advancements and future scientific discovery.
Challenges in Understanding Atomic Theory

Grasping the fundamental nature of matter has always posed a significant challenge for scientists. While remarkable progress has been made in understanding the building blocks of substances, there remain several obstacles that hinder a complete comprehension of how these components interact. These challenges stem from both the limitations of current experimental methods and the inherent complexities of the concepts themselves.
One of the primary difficulties in understanding the structure of matter is the scale at which these interactions occur. At the microscopic level, particles behave in ways that often defy classical intuition, making it hard to form clear, visualizable models. This abstract nature of subatomic particles, along with their wave-like properties, introduces a level of uncertainty that makes it challenging to describe their behavior with traditional language or concepts.
Furthermore, many of the interactions that occur at the smallest scales are governed by probabilistic rather than deterministic principles. This aspect of quantum mechanics, where outcomes can only be predicted in terms of probabilities, is counterintuitive to our everyday experiences and can be difficult for students and researchers alike to fully internalize.
Another major hurdle is the lack of direct observation. While advancements in particle accelerators and other experimental tools have allowed scientists to infer the properties of particles with great accuracy, direct observation of these fundamental components remains elusive. The inability to directly witness phenomena on such a small scale means that many concepts must rely on indirect evidence and mathematical models, which can sometimes create a sense of detachment from the physical reality they aim to explain.
Despite these challenges, ongoing research and technological advancements continue to push the boundaries of our understanding. As new methods are developed and our ability to simulate and observe the behavior of matter improves, the hope is that these barriers can be overcome, leading to even deeper insights into the nature of the universe.
Future Directions in Atomic Research
The pursuit of understanding the building blocks of matter is far from over. As technology advances, new frontiers in subatomic research are being explored, promising breakthroughs that could reshape our understanding of the universe. The next steps in this field are focused on refining existing theories, uncovering new particles, and developing more precise methods for studying the fundamental components of matter.
Advances in Particle Detection and Observation
One of the key areas of focus is improving particle detection technology. Current tools, like particle accelerators and detectors, have allowed scientists to make significant progress in understanding the properties and behaviors of fundamental components. However, there is still much to discover, especially in terms of understanding the behavior of extremely rare or transient particles. Future innovations in detector technology could lead to more accurate and comprehensive observations, enabling researchers to detect particles that were previously beyond our reach.
Exploring the Role of Quantum Computing
Quantum computing is poised to play a pivotal role in future research. By harnessing the unique properties of quantum bits, or qubits, scientists hope to simulate atomic and subatomic systems with unprecedented accuracy. This could allow for more efficient modeling of complex particle interactions, providing insights into phenomena that are currently difficult or impossible to simulate with classical computers. Quantum computers could significantly speed up the discovery process, potentially revealing new properties of matter and energy.
As research progresses, collaboration across disciplines–combining physics, mathematics, and computer science–will be essential. The goal is to not only enhance our theoretical understanding but also develop new technologies that can be applied across various scientific fields. The potential applications of these advancements are vast, from developing new materials and energy sources to revolutionizing medicine and computing.