
Understanding how energy is transferred and measured is crucial in the study of physical sciences. Experiments designed to observe the release of energy during combustion offer valuable insights into this process. In these hands-on activities, materials are ignited, and the resulting heat is measured to determine the energy content of various substances. By examining the reactions and using scientific tools, students gain a deeper understanding of how different materials release energy when burned.
The main goal of such experiments is to quantify the energy released from common items, offering a practical demonstration of fundamental scientific principles. These experiments typically involve a series of calculations that help us understand the relationship between heat, energy, and the materials being studied. Through this, participants can explore the efficiency of different materials and their potential uses in real-world applications.
Hands-on experience in these types of experiments not only reinforces theoretical knowledge but also builds critical thinking skills. As students collect and analyze data, they learn how to evaluate results, consider variables, and assess the accuracy of their measurements. This practical approach fosters a deeper appreciation for the scientific method and encourages curiosity about how everyday materials interact with energy.
Understanding the Energy Measurement Experiment
Experiments designed to measure the heat released from food items when ignited provide valuable insights into how energy is transferred during combustion. These practical activities serve as a way to demonstrate the relationship between energy and matter. By burning various substances, we can calculate the amount of heat produced and analyze the results to better understand the properties of these materials.
Key Concepts in the Experiment
The core idea behind this experiment is to observe how energy is released when a material undergoes combustion. This energy is measured using specific equipment, and the results help us calculate the total heat produced. These findings are then used to understand how efficiently the material releases energy. The main variables involved in these types of experiments are the type of substance being burned, the heat produced, and the energy transfer to the surrounding environment.
Energy Transfer and Efficiency
By measuring the heat produced and comparing it to the mass of the substance, we can determine the energy content per gram. This allows for comparisons between various materials and their potential applications. The efficiency of energy transfer is also a key factor in these experiments. How well the heat is captured and measured can impact the accuracy of the results and provide insights into ways to optimize the process.
| Material | Heat Released (cal/g) | Energy Efficiency |
|---|---|---|
| Sample 1 | 150 | 80% |
| Sample 2 | 120 | 75% |
| Sample 3 | 200 | 90% |
Exploring the Science of Energy Measurement
The study of energy release during combustion is a fundamental part of understanding how different materials produce heat. Through controlled experiments, we can observe and measure the amount of energy released when a substance undergoes combustion. This process provides critical insights into the relationship between energy, heat, and matter, forming the basis for various scientific and practical applications.
Energy measurement is not just about determining how much heat is released; it is also about understanding the efficiency of this energy transfer. By using specific tools and techniques, scientists can measure the energy output of different materials and compare their effectiveness in terms of heat production. These experiments allow for deeper exploration into how substances react to heat and the factors that influence their energy yield.
The key factors influencing the results include the type of material burned, the amount of heat it produces, and the efficiency with which the energy is captured and measured. By adjusting different variables, scientists can fine-tune their experiments to achieve more accurate and reliable data. This process also highlights the importance of precision and consistency in scientific measurement, ensuring that results can be applied to broader scientific questions.
Key Concepts in the Energy Measurement Experiment
The core principles behind experiments designed to measure energy release involve understanding the process of combustion and how heat is transferred from the material to its surroundings. These experiments aim to quantify the energy produced during the burning of substances and examine the efficiency of energy transfer. By grasping the fundamental concepts of heat production and measurement, we can better appreciate how different materials behave when ignited and how they release energy.
The Role of Heat Transfer
One of the main concepts explored in these experiments is the transfer of heat from the burning material to its environment. The heat generated during combustion can be measured using a variety of methods, such as calorimeters, which help track the amount of energy released. The efficiency of this energy transfer is a key factor in determining how much of the total heat is captured and how effectively it can be used for practical purposes.
Energy Calculations and Efficiency

In addition to measuring the total heat produced, these experiments often involve calculating the energy content of materials. By examining the mass of the substance burned and the temperature change of the surroundings, scientists can estimate the energy yield. This process also highlights the importance of efficiency–how much of the energy from combustion is captured versus lost in the process.
Purpose of the Energy Measurement Experiment
The primary goal of this experiment is to explore how different substances release energy when burned, and to understand the process of energy transfer during combustion. By conducting this experiment, we aim to observe and measure the heat produced by various materials, helping to uncover the efficiency of their energy release. This hands-on approach allows students to gain practical experience in the methods used to quantify energy, while reinforcing key scientific principles.
The main objectives of this activity include:
- Understanding the relationship between energy and matter during combustion
- Learning how to measure and calculate heat output from different materials
- Exploring the efficiency of energy transfer in various substances
- Examining the impact of different variables, such as mass and material composition, on energy release
Through this experiment, participants are also introduced to the concepts of energy efficiency and scientific accuracy, both of which are crucial in understanding how energy can be harnessed and utilized in real-world applications. By comparing the energy output of different materials, students can develop a deeper understanding of energy production and its practical uses in various industries.
How Snack Foods Are Used in Experiments
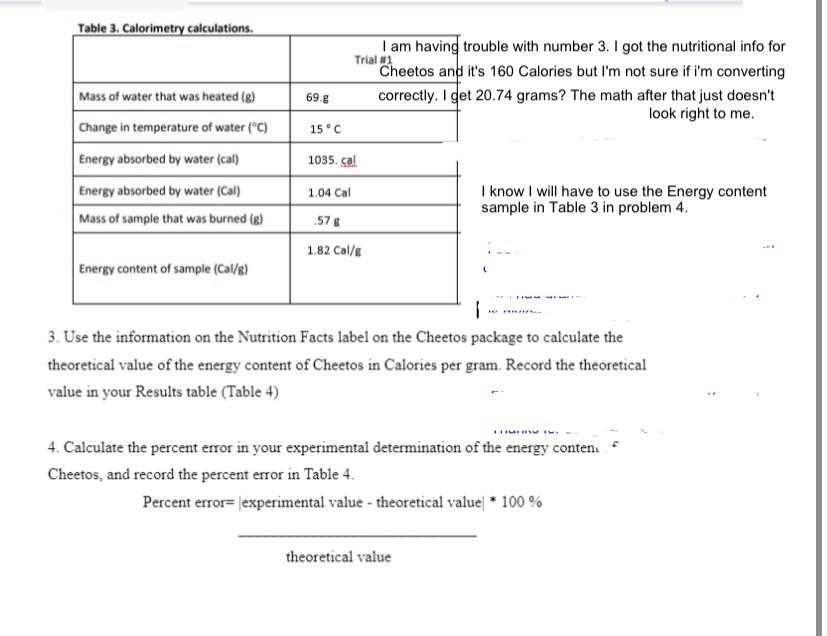
In energy measurement experiments, snack foods are commonly used as materials to demonstrate how substances release energy when burned. These foods, often rich in fats and carbohydrates, provide a practical way to observe and measure heat production during combustion. By using a familiar and easily obtainable material, the experiment becomes more accessible, helping students relate scientific principles to everyday items.
The process typically involves burning a piece of the snack in a controlled environment, where the energy released is captured and measured. The heat produced during the combustion is transferred to the surrounding environment, often through water or a similar medium, which allows for precise temperature changes to be recorded. This temperature change is then used to calculate the energy released by the food.
By using these materials in experiments, researchers can analyze the efficiency of energy transfer and how different substances compare in terms of energy output. This type of experiment is useful for understanding basic concepts in thermodynamics and provides students with a hands-on approach to learning about energy production and transfer.
The Role of Heat in Energy Measurement
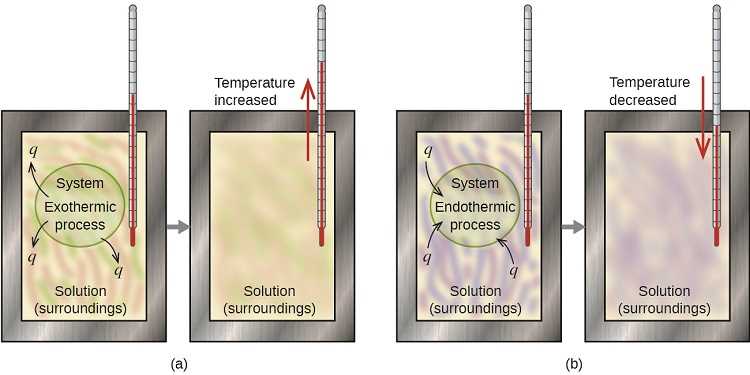
Heat plays a central role in experiments designed to measure the energy released by various substances during combustion. When a material is ignited, the chemical reaction produces energy in the form of heat, which is then transferred to the surroundings. This heat is crucial for understanding the efficiency and energy content of the material being tested. The transfer of heat allows scientists to calculate the amount of energy produced and evaluate how different materials compare in terms of energy yield.
In these types of experiments, the heat produced is typically measured by observing the temperature change in a substance like water. As the material burns and releases energy, the heat is absorbed by the surrounding medium, causing its temperature to rise. By accurately measuring this temperature change, researchers can determine the amount of energy released per unit of mass or volume of the substance being tested.
Understanding how heat is generated and transferred is essential for analyzing the results of these experiments. It helps to identify the energy content of different materials and allows for comparisons between substances with varying properties. Heat measurement is not only a fundamental concept in thermodynamics but also a key tool in assessing the practical applications of energy sources in real-world scenarios.
Basic Principles of Energy Transfer

Energy transfer refers to the movement of energy from one system or object to another. In many scientific experiments, understanding how energy flows from a material undergoing combustion to its surroundings is crucial for accurate measurements. This process involves the conversion of chemical energy into heat, which is then transferred to a surrounding medium like water or air. The ability to track and quantify this transfer is fundamental for studying energy production and efficiency.
Types of Energy Transfer
There are three primary mechanisms through which energy can be transferred: conduction, convection, and radiation. Each of these methods plays a role in how heat is transferred from a burning material to its environment. Conduction occurs when heat flows through a material, while convection involves the movement of heat through fluids like air or water. Radiation, on the other hand, refers to the emission of energy as electromagnetic waves, which can travel through space without the need for a medium.
Efficiency of Energy Transfer

Efficiency refers to how effectively energy is transferred from one system to another. In energy measurement experiments, maximizing the efficiency of energy transfer is essential for obtaining accurate results. Factors such as the temperature gradient, the medium used for energy transfer, and the material being burned all impact the efficiency. By optimizing these factors, scientists can ensure that the energy measured reflects the true energy content of the material being tested.
Methods of Measuring Heat Production
Accurately measuring heat production during experiments is crucial for understanding the energy content of various materials. Several methods are commonly used to quantify the amount of heat released during combustion or other energy-producing processes. These techniques involve capturing and recording temperature changes in a controlled environment to estimate the total heat output of the material being tested.
The most common methods include:
- Calorimeters: Devices designed to capture and measure the heat released from a substance. Calorimeters use water or another medium to absorb the heat, and the temperature change is used to calculate energy output.
- Temperature Probes: These tools measure the temperature of a substance before and after it undergoes combustion. The difference in temperature helps determine how much energy has been released.
- Heat Flow Meters: Instruments that measure the rate at which heat flows through a material. These meters provide insights into the speed and efficiency of heat transfer during the experiment.
In addition to these direct measurement tools, indirect methods may also be employed, such as calculating energy based on the mass of the material burned and the specific heat capacity of the surrounding medium. By combining multiple measurement techniques, researchers can improve the accuracy and reliability of their results.
Calculating Energy from Combustion
In experiments designed to measure energy output from combustion, the amount of heat released can be calculated by measuring the temperature change in a surrounding medium, typically water. This process involves determining the heat absorbed by the water and using that information to estimate the energy produced by the substance being burned. The calculation is based on the relationship between heat, mass, specific heat capacity, and temperature change.
To calculate the energy released from the combustion of a material, the following formula is commonly used:
Q = mcΔT
- Q: Energy released (in joules)
- m: Mass of the water (in grams)
- c: Specific heat capacity of water (4.18 J/g°C)
- ΔT: Change in temperature of the water (in °C)
By knowing the temperature change and the mass of the water, the amount of heat absorbed can be calculated. From there, the energy produced by the burning material can be determined. Below is an example of a table used to calculate the energy released during the combustion process:
| Parameter | Value | Unit |
|---|---|---|
| Mass of water (m) | 100 | grams |
| Specific heat capacity (c) | 4.18 | J/g°C |
| Temperature change (ΔT) | 5 | °C |
| Energy released (Q) | 2090 | joules |
In this example, the energy released by the material during combustion is calculated as 2090 joules. This method allows for a precise quantification of the energy output, which can be used to compare the energy content of different substances.
Understanding Calorimeter Functions

Calorimeters are essential instruments in experiments designed to measure heat released during a chemical reaction or combustion process. These devices are specifically designed to capture the heat produced by a material and measure how much energy is transferred to the surrounding medium, typically water. By accurately tracking the temperature changes, calorimeters provide valuable data that helps researchers quantify the energy output of different substances.
How Calorimeters Work
A calorimeter works by isolating the reaction that produces heat and preventing the loss of energy to the environment. The substance being tested is burned or reacted in a controlled chamber, and the heat it generates is transferred to a surrounding medium, often water. The calorimeter is equipped with temperature sensors that measure the rise in temperature of the surrounding medium. The amount of heat energy is then calculated based on the temperature change, the mass of the medium, and its specific heat capacity.
Types of Calorimeters
There are various types of calorimeters, each suited to different types of experiments and materials. The most commonly used types include:
- Bomb Calorimeter: This is used for measuring the heat of combustion of a substance. The material is placed in a sealed container, and the heat produced by its burning is absorbed by the surrounding water.
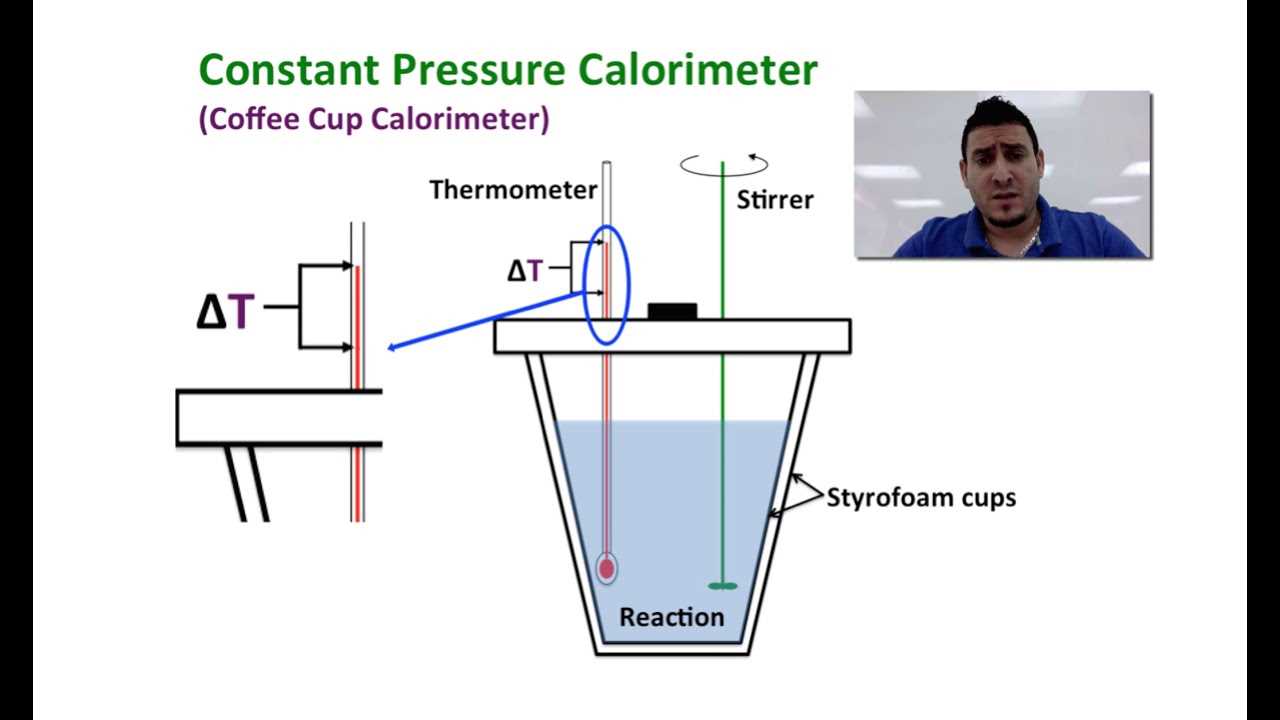
- Coffee Cup Calorimeter: A simpler design, this type is often used for solutions in liquid form. It consists of a styrofoam cup to minimize heat loss and is ideal for measuring reactions in aqueous solutions.
- Differential Scanning Calorimeter (DSC): This method is used to study phase transitions in materials, such as melting or crystallization. The DSC measures the heat flow into or out of the sample as its temperature is controlled.
Understanding the different types of calorimeters and their functions is crucial for selecting the right tool for the experiment at hand. Each type is designed to optimize the accuracy and efficiency of measuring heat transfer and energy production in various scientific contexts.
Accuracy in Heat Measurement Results
When measuring the heat produced during a reaction or combustion process, achieving accurate results is crucial for ensuring reliable data. Several factors can influence the precision of the measurements, including the experimental setup, environmental conditions, and the calibration of the instruments used. Understanding these variables and how to minimize errors is essential for obtaining consistent and trustworthy results.
Key Factors Affecting Accuracy
There are several factors that can impact the accuracy of heat measurement results:
- Heat Loss: Inaccurate results can occur if heat escapes from the system during the experiment. Ensuring the system is insulated properly can help minimize this loss.
- Calibration: Calorimeters and other measuring instruments must be calibrated correctly before use. Without proper calibration, the readings may be skewed and lead to incorrect conclusions.
- Measurement Errors: Errors in measuring the mass of the substance being tested or the temperature of the medium can introduce inaccuracies into the calculation. Using precise instruments and taking multiple readings can help reduce these errors.
Improving Accuracy in Results

To improve the accuracy of heat measurement experiments, several steps can be taken:
- Use Insulated Containers: To prevent heat loss, experiments should be conducted in well-insulated containers. This ensures that the majority of the heat is absorbed by the surrounding medium.
- Perform Multiple Trials: Conducting multiple trials of the same experiment allows for averaging out any anomalies or errors in individual readings.
- Control External Variables: Temperature fluctuations, air currents, and humidity can affect results. Performing the experiment in a controlled environment can help ensure consistency.
By understanding the factors that affect accuracy and taking steps to minimize errors, researchers can obtain reliable and meaningful data from their heat measurement experiments.
Common Challenges in Heat Measurement Experiments
Heat measurement experiments can provide valuable insights into the energy content of materials, but they often come with a range of challenges that can affect the accuracy and reliability of the results. From environmental factors to technical limitations, understanding these common obstacles can help researchers improve the design and execution of their experiments. Identifying and addressing these issues is key to obtaining trustworthy data in any energy measurement study.
Environmental Factors
External conditions can significantly impact the outcomes of energy measurement experiments. Factors such as temperature fluctuations, air drafts, and humidity can lead to heat loss or gain that is not accounted for in the results. For instance, a sudden drop in room temperature can cause the surrounding medium to absorb less heat, skewing the final readings. To mitigate these effects, experiments are often conducted in controlled environments or with additional insulation to minimize temperature variation.
Instrumental Limitations
Another major challenge in heat measurement studies is the accuracy of the instruments used. Even the most advanced devices can have inherent limitations, such as sensitivity issues, calibration errors, or inconsistencies in readings. Instruments must be carefully calibrated before each experiment to ensure precise measurements. Failure to do so can result in data that is difficult to interpret or compare accurately across trials. Regular maintenance and calibration of measuring equipment are essential for obtaining reliable results.
By recognizing and addressing these challenges, researchers can enhance the quality of their experiments and draw more accurate conclusions from their data. Taking steps to control external variables and ensuring the proper functioning of measurement devices are fundamental to achieving success in energy analysis experiments.
Applications of Heat Measurement in Science
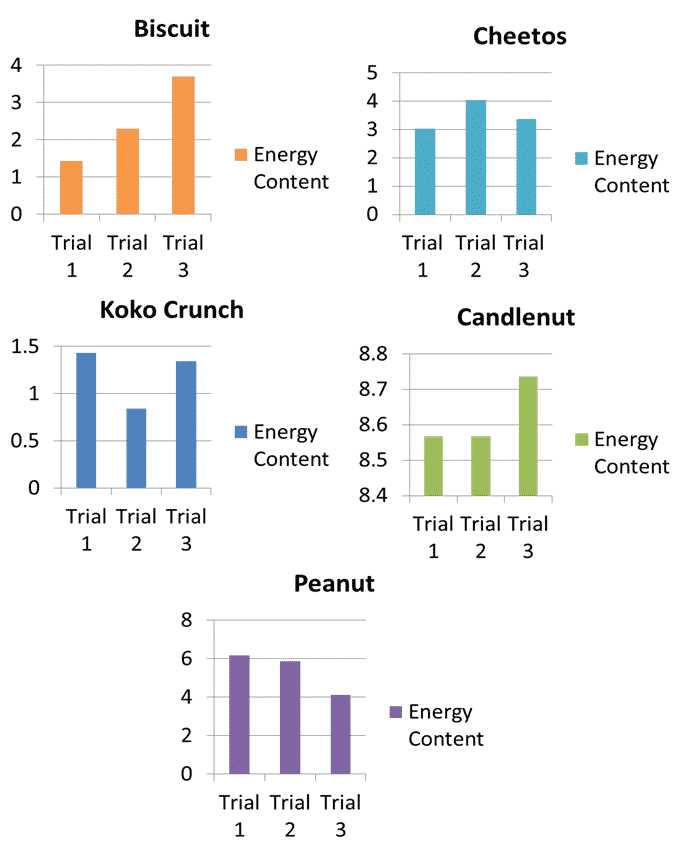
Heat measurement plays a crucial role in a wide range of scientific fields, offering insights into the energy dynamics of various substances and processes. By quantifying the heat produced or absorbed during chemical reactions, physical changes, or biological processes, researchers are able to understand underlying mechanisms, optimize systems, and develop new technologies. The versatility of energy measurement extends from basic research to practical applications in multiple industries.
In chemistry, heat measurement helps to determine the energy content of different compounds, guiding the development of new materials, fuels, and chemical processes. In biology, this technique is used to study metabolic reactions, including the energy expenditure of organisms. In environmental science, it is applied to understand energy flows in ecosystems and the impact of human activities on natural processes.
Moreover, in engineering, accurate energy measurements are essential for improving the efficiency of machines, engines, and electronic devices. In food science, measuring the caloric content of different foods through energy release during combustion helps determine their nutritional value. These diverse applications highlight the importance of heat measurement in advancing our knowledge and improving technologies across various scientific domains.
What Affects the Results of the Experiment
The accuracy and reliability of any experiment depend on a variety of factors, both controllable and uncontrollable. Understanding these influences is crucial for interpreting data correctly and improving experimental outcomes. While some variables can be minimized or eliminated through careful planning, others may need to be accounted for as potential sources of error. Below are some of the key factors that can affect the results of energy measurement experiments.
Environmental Factors
The conditions in which an experiment is conducted can have a significant impact on the results. Variations in temperature, humidity, and air pressure can all influence the energy transfer during an experiment. For instance, if the surrounding environment is too cold, heat may be lost to the surroundings, skewing the results. Similarly, fluctuating room temperatures or drafts can cause inconsistent readings, leading to inaccurate conclusions.
- Temperature: Heat loss or gain from the environment can affect measurements.
- Humidity: High moisture levels can alter the behavior of substances being tested.
- Airflow: Drafts or ventilation can cause changes in temperature readings.
Instrumental and Methodological Issues

The equipment used in the experiment plays a vital role in the accuracy of the data collected. Inaccurate calibration or malfunctioning instruments can lead to erroneous readings. Furthermore, the methodology itself can introduce errors. For example, improper handling of materials, contamination, or inconsistent timing can all lead to unreliable results. Careful calibration of instruments and strict adherence to standardized procedures are essential to mitigate these risks.
- Calibration: Incorrect calibration can lead to inaccurate heat measurements.
- Measurement technique: Variations in how measurements are taken can introduce errors.
- Instrument malfunctions: Faulty equipment may lead to unreliable data.
By considering and addressing these variables, researchers can improve the precision of their experiments, ultimately leading to more consistent and trustworthy results.
Implications of Energy Measurement for Energy Studies
Understanding how energy is transferred and measured is fundamental to various fields of scientific research, especially in the study of energy sources and consumption. The ability to quantify energy content allows researchers and industries to better evaluate the efficiency of fuels, materials, and processes. This section explores the broader implications of energy measurement in scientific studies and its role in shaping sustainable practices and technological advancements.
Enhancing Energy Efficiency
Accurate energy measurements help identify the most efficient energy sources and conversion methods. By understanding how much energy is produced by different materials or processes, researchers can improve the design of energy systems. This can lead to innovations in energy production, storage, and usage, as well as the development of technologies that maximize energy output while minimizing waste.
- Improved fuel efficiency: Identifying high-energy materials leads to better fuel choices.
- Optimized energy conversion: Ensuring maximum energy extraction from raw sources.
- Waste reduction: Reducing unnecessary energy loss in various systems.
Impacts on Sustainability and Environmental Studies
The study of energy production and its environmental impact is essential for addressing sustainability challenges. By accurately measuring the energy content of different substances, scientists can assess their environmental footprint and develop strategies to minimize the use of harmful resources. This data is also vital in evaluating the feasibility of renewable energy sources, such as biofuels and solar power, in comparison to traditional fossil fuels.
- Environmental footprint: Understanding energy sources’ effects on the environment.
- Renewable energy potential: Assessing the energy output of green alternatives.
- Carbon footprint reduction: Reducing emissions by identifying cleaner energy options.
Incorporating energy measurement into these studies not only contributes to scientific understanding but also supports policy-making and decision-making processes aimed at a more sustainable future.