Mastering the basic principles of atomic connections is essential for anyone studying chemistry. The way atoms come together to form compounds involves a series of steps that determine their properties and behavior. This process, which is foundational to molecular science, requires a clear understanding of how electrons are shared or transferred between atoms.
In this guide, we will explore the methods and techniques used to represent these atomic interactions visually. These visual aids serve as tools for scientists to predict molecular shapes, bonding patterns, and the overall stability of a compound. By learning how to interpret and create these visual representations, you can unlock deeper insights into chemical behavior and molecular geometry.
With a focus on key concepts like electron distribution, molecular shapes, and bonding principles, this article will provide you with a structured approach to understanding how atoms link up to form stable compounds. Each section will guide you through different aspects of this topic, helping you build a strong foundation in molecular chemistry.
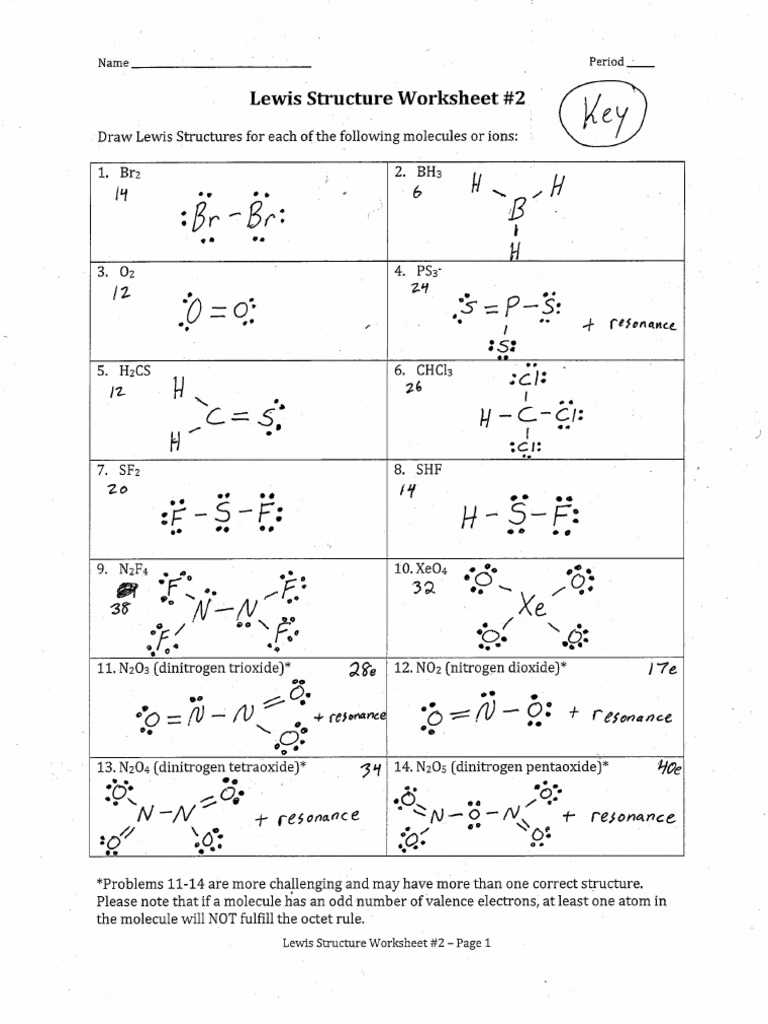
Chemquest 14 Molecular Bonding Solutions
Understanding the connections between atoms is a fundamental aspect of chemistry. This section delves into the methods used to visually represent these atomic interactions, focusing on how electrons are arranged and shared to form stable molecules. By exploring these models, students can gain a deeper understanding of molecular behavior and predict how atoms will bond in various compounds.
The task involves interpreting specific examples where atoms combine, analyzing the placement of electrons around each atom, and identifying the overall molecular shape. These models help to visualize the balance between different atomic forces, providing clarity on the nature of chemical bonds and how they influence the properties of substances. The exercise aims to improve your ability to predict molecular characteristics and enhance your understanding of bonding principles.
Throughout this section, we will break down the process of constructing these diagrams step by step. By applying the rules of electron sharing and bonding, you will be able to effectively illustrate how atoms bond to create molecules with distinct shapes and characteristics. The focus will be on accuracy and a methodical approach to solving these chemical puzzles, which are essential for mastering molecular chemistry.
Understanding Molecular Bonding Diagrams
In the study of chemistry, visual representations of atomic interactions are crucial for understanding how elements combine to form compounds. These representations, which highlight the sharing of electrons between atoms, provide valuable insights into molecular shapes, bonding types, and the overall behavior of substances. Learning to interpret these diagrams is essential for anyone seeking to grasp the fundamentals of molecular chemistry.
The Role of Electron Pairs in Bonding
One of the core concepts in these diagrams is the placement of electron pairs around atoms. These pairs represent the electrons involved in forming chemical bonds, and their arrangement dictates the molecule’s shape and properties. Understanding how to place and interpret these pairs is vital for drawing accurate models.
- Valence electrons are crucial for determining bonding behavior.
- Shared pairs of electrons form covalent bonds between atoms.
- Unshared electrons, or lone pairs, affect the molecular geometry.
Steps to Drawing Molecular Bonding Models
To accurately represent the interactions between atoms, follow a systematic approach. This ensures that each bond is drawn with precision, adhering to the fundamental rules of electron distribution.
- Determine the total number of valence electrons for the atoms involved.
- Place the atoms in a way that reflects their bonding preferences.
- Distribute the electrons, starting with bonding pairs, and then add lone pairs as needed.
- Verify that each atom satisfies the octet rule, except for hydrogen and helium.
By mastering these steps, you can accurately depict the bonding relationships within molecules, enhancing your understanding of molecular chemistry and its applications in real-world contexts.
Importance of Bonding in Chemistry
In chemistry, understanding how atoms interact to form molecules is fundamental to grasping the behavior of matter. The way atoms bond determines the physical and chemical properties of the substances they create, influencing everything from the state of matter to reactivity and stability. Bonding is the key to understanding how molecules behave in different environments, making it a crucial concept in both theoretical and applied chemistry.
Types of Chemical Bonds

There are various types of bonds formed between atoms, each with distinct characteristics. These bonds define the strength and stability of the resulting compounds, and understanding them helps predict how substances will react under different conditions.
- Covalent bonds occur when atoms share electrons, leading to the formation of stable molecules.
- Ionic bonds involve the transfer of electrons from one atom to another, creating charged particles called ions.
- Metallic bonds are formed when metal atoms share their electrons in a “sea” of electrons, giving metals their unique properties.
Bonding and Molecular Properties

The type of bond between atoms has a direct impact on the properties of the molecules they form. For example, substances with covalent bonds tend to have lower melting points and are often insulators, while ionic compounds have high melting points and conduct electricity in liquid form. By understanding the nature of bonds, chemists can predict the behavior of substances in various chemical reactions, making bonding a key concept in material science, biology, and industrial applications.
Step-by-Step Guide to Drawing Molecular Bonding Diagrams
Creating accurate molecular models is essential for understanding how atoms interact and bond to form compounds. These diagrams help visualize the arrangement of electrons and the way atoms are connected within a molecule. By following a structured approach, you can effectively represent how atoms share or transfer electrons, which is crucial for predicting molecular shapes and chemical behavior.
Below is a step-by-step guide to drawing these bonding diagrams, ensuring that you account for all valence electrons and adhere to key bonding principles.
Step 1: Count the Total Valence Electrons
The first step in constructing a bonding model is to determine the total number of valence electrons in the molecule. Valence electrons are the outermost electrons involved in bonding. Add the valence electrons of each atom involved in the molecule. For example, oxygen has six valence electrons, and hydrogen has one. Make sure to adjust the count for charged molecules by adding or subtracting electrons based on the charge.
Step 2: Arrange the Atoms
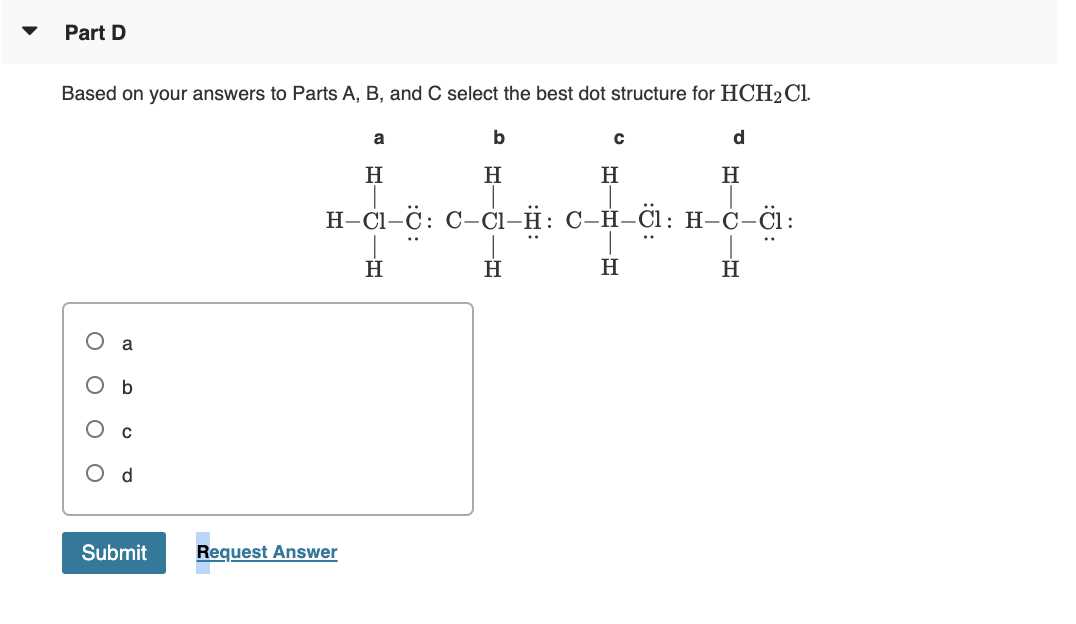
Once you know the number of valence electrons, arrange the atoms to reflect how they will bond. Typically, the atom with the lowest electronegativity will be placed in the center. For example, in a molecule of water (H₂O), oxygen will be the central atom, with the hydrogen atoms bonded to it. Always place atoms in a way that reflects their typical bonding preferences and valency.
Step 3: Form Bonds Between Atoms
Next, begin connecting the atoms by placing pairs of electrons between them to form bonds. A single bond consists of two electrons shared between two atoms. Start by creating single bonds between the atoms, and check if all atoms are connected in a way that satisfies their bonding requirements.
Step 4: Distribute Remaining Electrons
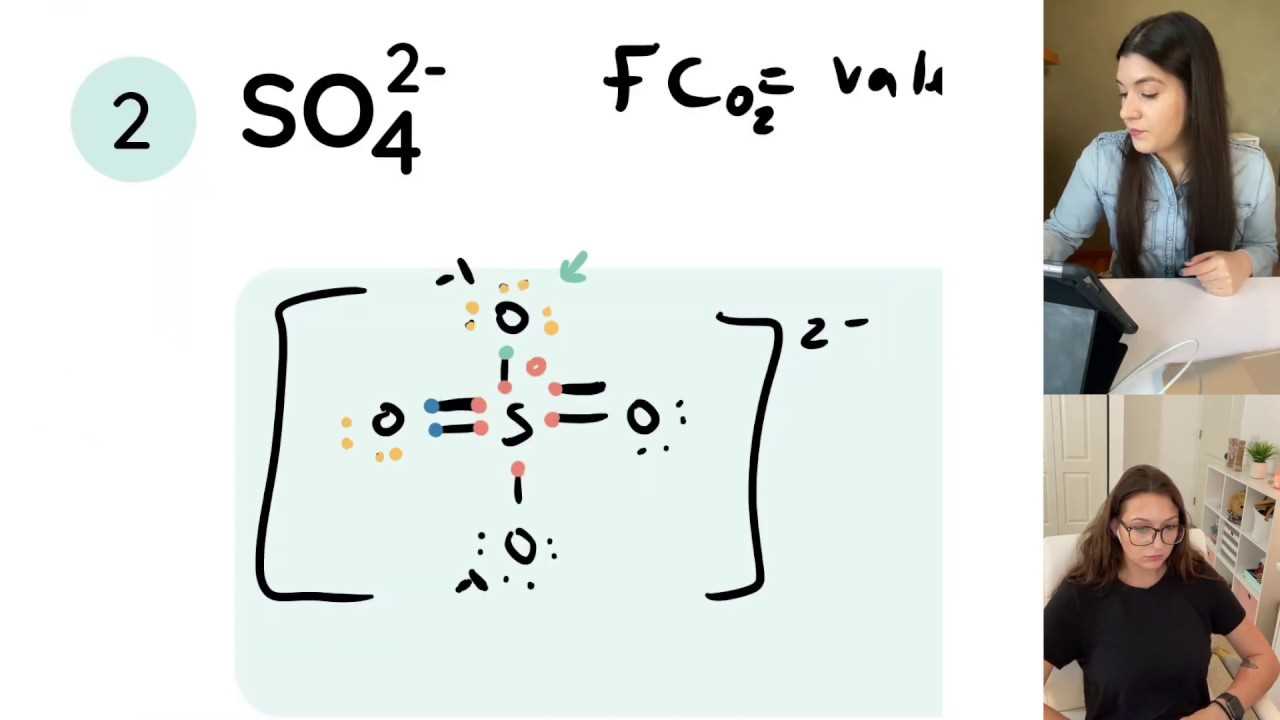
After forming bonds, place the remaining electrons around the atoms as lone pairs. These are the electrons that are not involved in bonding. Distribute them to fill the octet for each atom, starting with the most electronegative atoms. If there are not enough electrons to satisfy the octet rule, consider forming double or triple bonds.
Step 5: Check for the Octet Rule
Ensure that each atom, except for hydrogen, has a full octet of electrons (8 electrons around it). If an atom does not have enough electrons, adjust by moving lone pairs to form additional bonds. This step is crucial for achieving stability in the molecule.
By following these steps, you can create an accurate representation of molecular bonding, giving you a better understanding of chemical interactions and molecule stability.
Common Mistakes in Molecular Bonding Diagrams
Creating accurate bonding models can be challenging, especially when trying to represent complex molecular structures. Common errors can lead to incorrect diagrams that fail to capture the true nature of atomic interactions. These mistakes can affect the predicted properties of molecules, so it is essential to recognize and correct them when drawing bonding diagrams.
Incorrect Electron Count
One of the most common mistakes in molecular diagrams is incorrectly counting the total number of valence electrons. Failing to account for the correct number can lead to incorrect bonding and the wrong molecular geometry. Be sure to carefully add up the electrons for each atom and adjust the count for any ions or charged molecules. Remember, when dealing with negative ions, add electrons; for positive ions, subtract electrons.
Violating the Octet Rule
Another frequent error is not adhering to the octet rule, where atoms fail to obtain a stable electron configuration of eight electrons (except for hydrogen, which follows the duet rule). Some diagrams may result in atoms with too few or too many electrons, which can make the molecule unstable or unrealistic. Always check that the atoms are correctly surrounded by electrons and adjust bonds when necessary.
By avoiding these common mistakes and following the proper procedures for drawing bonding models, you can ensure your diagrams accurately represent the molecular structure and behavior of the substances you’re studying.
Electron Configuration and Molecular Bonding Diagrams
Electron configuration plays a crucial role in understanding how atoms bond and form molecules. The arrangement of electrons in an atom determines its reactivity and bonding behavior. These configurations help predict how atoms will interact with one another to form stable structures. The way electrons are distributed around atoms is essential for constructing accurate molecular bonding diagrams, as it dictates the number of bonds and the overall shape of the molecule.
The Role of Valence Electrons
Valence electrons, the electrons in the outermost shell of an atom, are the most involved in bonding. These electrons participate in the formation of bonds with other atoms, either by sharing or transferring electrons. Understanding the electron configuration helps identify how many valence electrons an atom has and how they will be arranged in a bonding model. The number of valence electrons is key to determining how atoms connect to one another and form stable compounds.
From Electron Configuration to Bonding Models

Once the electron configuration is known, it can be used to draw bonding diagrams that represent the electron-sharing process. The configuration guides the placement of electrons in the model, ensuring that each atom achieves a stable arrangement, typically by filling its valence shell. This is crucial for creating accurate diagrams that show the true nature of the molecule and how atoms interact within the structure. Understanding the connection between electron configuration and bonding models is essential for predicting molecular properties and behavior.
How to Predict Molecular Shapes
Predicting the shape of a molecule is a crucial step in understanding its properties and behavior. The arrangement of atoms in a molecule determines its physical and chemical characteristics, such as polarity, reactivity, and how it interacts with other molecules. By understanding the factors that influence molecular geometry, it is possible to predict the three-dimensional shape of a molecule even before conducting experiments.
The key to predicting molecular shapes lies in understanding how atoms are bonded and how lone pairs of electrons influence the geometry. The theory behind this involves the repulsion between electron pairs, which leads to specific spatial arrangements. By applying certain principles, such as the Valence Shell Electron Pair Repulsion (VSEPR) theory, we can predict the geometry of a molecule based on the number of bonding and lone electron pairs around the central atom.
Steps for Predicting Molecular Geometry
To predict the shape of a molecule, follow these steps:
- Step 1: Count the total number of valence electrons for all atoms involved in the molecule.
- Step 2: Determine how many bonding and lone pairs of electrons are present around the central atom.
- Step 3: Apply the VSEPR theory to predict how these pairs will arrange themselves to minimize repulsion.
- Step 4: Use the resulting electron pair arrangement to deduce the molecular shape.
Common Molecular Geometries
Based on the number of bonding pairs and lone pairs around the central atom, several distinct molecular shapes can occur. Some common examples include:
- Linear: Occurs when there are two bonding pairs and no lone pairs, leading to a straight-line geometry.
- Trigonal Planar: Occurs with three bonding pairs and no lone pairs, forming a flat triangular shape.
- Tetrahedral: Occurs with four bonding pairs, creating a three-dimensional shape.
- Trigonal Bipyramidal: Results from five bonding pairs, forming a structure with axial and equatorial positions.
- Octahedral: Occurs when there are six bonding pairs, creating a symmetrical, three-dimensional shape.
By following these steps and understanding the principles behind molecular geometry, you can predict the shape of nearly any molecule and gain insights into its chemical behavior and interactions.
Formal Charge and Molecular Diagrams
In the study of chemical bonding, understanding the distribution of electrons within a molecule is crucial for predicting its stability and reactivity. The concept of formal charge helps in assessing how electrons are allocated between atoms, allowing chemists to determine the most stable arrangement of atoms within a molecule. By evaluating the formal charge, one can identify the most favorable bonding patterns and the best configuration for the atoms involved, leading to a better understanding of the molecule’s behavior.
Formal charge is a theoretical calculation used to determine the electron distribution within a molecule, based on the assumption that electrons in bonds are shared equally. The formal charge helps predict how atoms interact and allows for the identification of potential areas where electron density may be uneven. A molecule’s stability is often directly related to minimizing the formal charges on the individual atoms involved.
To calculate the formal charge, consider the number of valence electrons an atom would normally have in its neutral state, the number of electrons assigned to it in the molecule, and the number of bonds it forms. By applying this formula, it is possible to determine the charge of an atom and make decisions about the most stable arrangement of atoms in the molecule.
The ideal molecule structure minimizes the formal charge across all atoms, as this typically corresponds to the most stable and energetically favorable configuration. By considering formal charge, chemists can make predictions about molecular geometry, polarity, and reactivity, which are essential for understanding chemical reactions and designing new compounds.