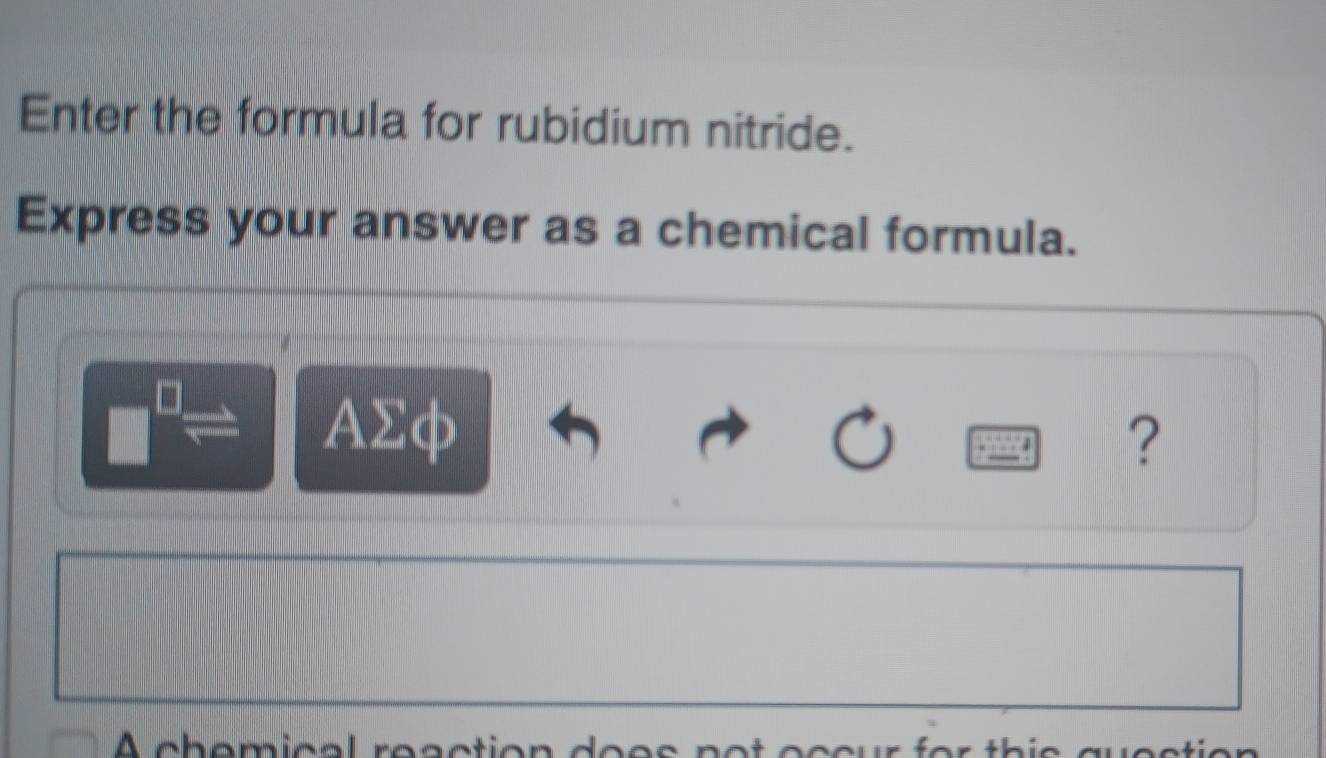
Conveying scientific relationships often requires precision and clarity. In the world of science, symbolic representation plays a crucial role in simplifying complex ideas into universally understood formats.
Learning how to use these representations effectively helps bridge theoretical concepts and practical applications. By understanding their structure and meaning, you can unlock a deeper comprehension of interactions between various components.
In this guide, we will explore strategies to decode and construct these notations. With clear examples and straightforward steps, the process becomes accessible to learners of all levels.
Understanding Chemical Formulas in Simple Terms
Symbols and numbers are fundamental tools in describing the composition of substances. They provide a concise way to represent the structure and proportions of elements, making it easier to study their interactions and properties.
The Role of Symbols in Science
Each element is identified by a unique abbreviation, derived from its name or historical origin. These abbreviations simplify communication and ensure that the same information is understood across different languages and fields of study.
Numbers That Define Relationships
Quantitative details, such as ratios and counts, are often included to specify the exact nature of a compound. These values are critical for distinguishing between substances with similar components but different structures or behaviors.
By mastering these basic principles, interpreting and utilizing scientific notations becomes a straightforward and practical skill.
Key Components of a Chemical Formula
Representations in science rely on a combination of symbols and numerical values to convey essential information about a substance. These elements work together to provide clarity and accuracy in describing compositions.
The symbols used are abbreviations for fundamental units, while numbers indicate their specific quantities. Together, they form a complete depiction of a compound’s makeup, allowing researchers and learners to understand its structure and behavior.
By focusing on these foundational aspects, one can build a strong understanding of how substances are represented and how their interactions are studied.
Steps to Create Accurate Chemical Formulas
Creating precise representations of substances involves following a logical sequence. This ensures that each part of the structure is correctly identified and accurately balanced. By breaking the process into smaller steps, even complex tasks become manageable.
- Identify the components: Determine all elements or groups involved in the substance. Ensure you understand their standard symbols and roles.
- Determine ratios: Establish how the parts combine by analyzing their proportions. Use data from experiments or established rules.
- Balance the structure: Adjust quantities to maintain consistency with established laws, such as charge neutrality or stoichiometric principles.
- Review for accuracy: Cross-check the results to confirm correctness. Verify that the representation matches the intended composition.
Following these steps ensures that the representation not only communicates effectively but also aligns with scientific principles.
How Elements Combine in Chemistry
The combination of different elements forms the foundation of countless substances found in nature and created in laboratories. These unions follow specific rules that govern the behavior and interactions of particles, resulting in stable structures.
Types of Bonds Between Particles
Interactions between particles can vary based on their properties, leading to distinct types of connections. Understanding these relationships is key to predicting how substances form and behave.
| Type of Interaction | Characteristics |
|---|---|
| Ionic Bond | Formed through the transfer of particles, creating charged species that attract each other. |
| Covalent Bond | Occurs when particles share pairs to achieve stability, often resulting in neutral structures. |
| Metallic Bond | Involves a shared “sea” of particles in a network, giving rise to unique properties like conductivity. |
Key Factors Influencing Combinations

Several factors, such as energy levels and spatial arrangements, determine how particles interact. Recognizing these influences helps explain why some combinations are more common or stable than others.
Interpreting Symbols and Numbers in Formulas

Representations in science rely on a system of abbreviations and numerical values to convey detailed information about substances. These components work together to describe the makeup and behavior of compounds with precision.
Understanding Abbreviations
Each symbol corresponds to a specific particle or group. Derived from standardized naming conventions, these abbreviations make it easy to identify and communicate the components of a substance across different contexts. For example, H stands for hydrogen, while O represents oxygen.
The Role of Numbers
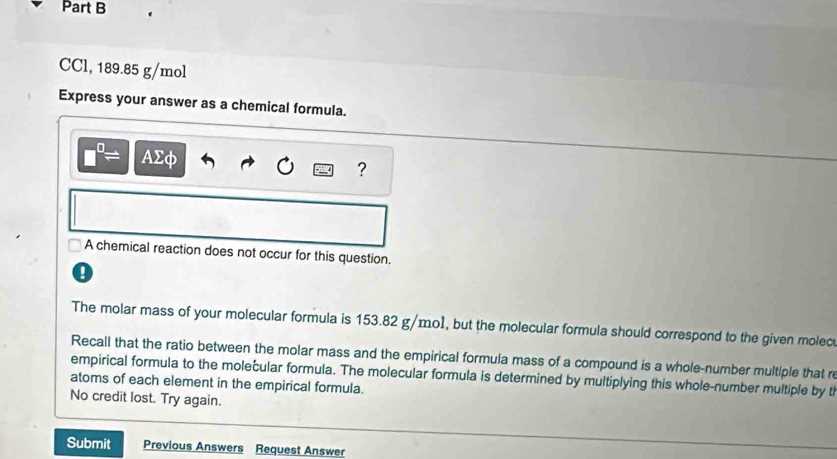
Numerical values play a crucial role in defining proportions and relationships. Small numbers written below the line, called subscripts, indicate the quantity of a particular component in a unit. Numbers written before a symbol, known as coefficients, show how many units are involved in a reaction or process.
By understanding the meaning behind these symbols and values, one can interpret and use scientific notations more effectively in practical applications.
Common Mistakes in Writing Formulas
When creating representations of substances, it’s easy to make errors that can lead to misinterpretation or confusion. Recognizing common mistakes helps improve accuracy and ensures that the correct information is conveyed.
Frequent Errors in Symbol Use
- Incorrect capitalisation: Symbols must follow strict capitalization rules. For example, o is not the same as O, as the former doesn’t represent oxygen.
- Using outdated or incorrect abbreviations: Always use the proper abbreviations for elements, such as Na for sodium, instead of an incorrect form.
- Confusing similar symbols: Some symbols are very similar in appearance but represent different elements, such as C for carbon and Cl for chlorine.
Misinterpreting Numbers
- Incorrect placement of numbers: Numbers should be placed as subscripts to indicate quantity. Placing them incorrectly can result in a misrepresentation.
- Omitting necessary values: Failing to include important numerical values can lead to incomplete or inaccurate descriptions.
- Confusing coefficients and subscripts: Coefficients represent the number of molecules, while subscripts show the number of atoms. Confusing these can lead to incorrect calculations.
Avoiding these common mistakes ensures that the substance’s composition is accurately represented, allowing for clearer communication and better understanding.
The Importance of Subscripts in Chemistry
Subscripts play a critical role in representing the composition of substances accurately. They help specify the exact number of particles involved in a particular compound, ensuring precise communication in scientific contexts.
Defining Proportions and Quantities
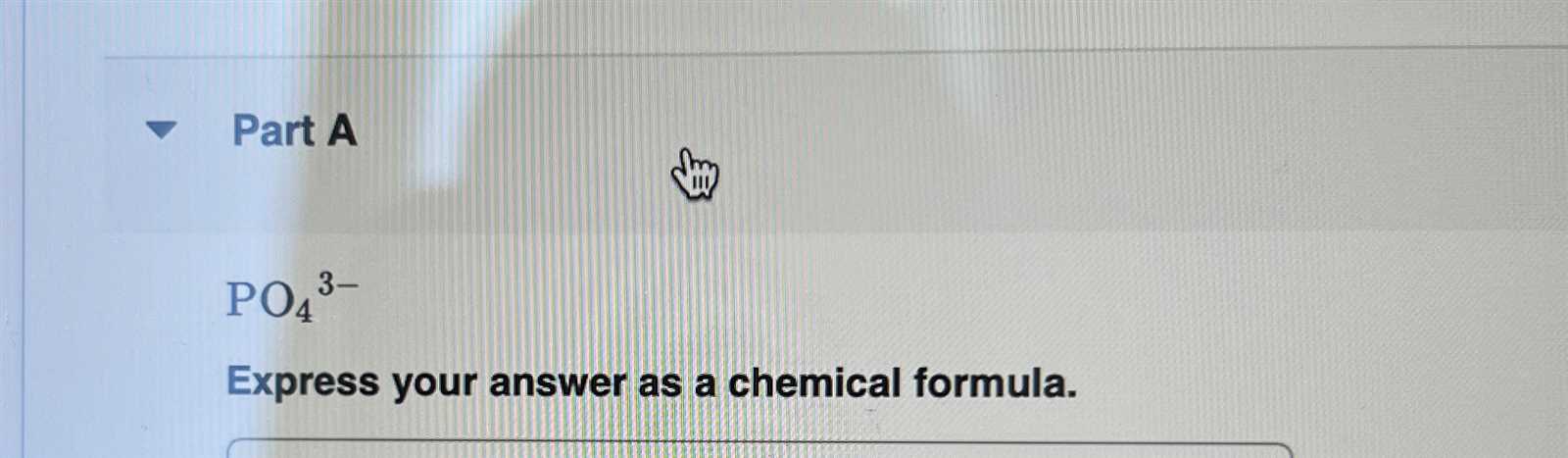
By using subscripts, scientists can indicate how many atoms of each element are present in a molecule. For example, in water (H2O), the subscript “2” signifies that two hydrogen atoms are combined with one oxygen atom. This allows for a clear understanding of the substance’s structure and behavior.
Enhancing Precision in Chemical Reactions
Subscripts also contribute to the accuracy of chemical reactions. By defining the exact ratio of atoms involved, subscripts ensure that reactions are balanced correctly, making it possible to predict the outcome with reliability. Whether in laboratory work or theoretical calculations, subscripts are indispensable for maintaining precision.
Examples of Real-World Chemical Formulas
Understanding how substances are represented in scientific notation is essential for identifying their properties and uses. Below are examples of how common compounds and elements are expressed in this format, showing their real-world applications.
| Substance | Representation | Common Use |
|---|---|---|
| Water | H2O | Used for drinking, cooking, and industrial processes. |
| Carbon Dioxide | CO2 | Vital for plant photosynthesis and a greenhouse gas. |
| Sodium Chloride | NaCl | Commonly used as table salt for seasoning and preserving food. |
| Ammonia | NH3 | Used in fertilizers and cleaning products. |
| Glucose | C6H12O6 | An essential sugar used by the body for energy. |
These examples demonstrate how representations provide a shorthand method of conveying complex information about substances, helping scientists and industries communicate more efficiently.
Difference Between Empirical and Molecular Formulas
There are various ways to represent the composition of a substance, and two common methods are through simplified and exact representations of the quantities of atoms involved. While both types serve to describe the same substance, they provide different levels of detail.
Empirical Formulas
Empirical representations give the simplest ratio of elements within a compound, showing the smallest whole-number ratio between the atoms of each element. For example, the empirical formula for glucose (C6H12O6) is CH2O, which simplifies the composition without showing the actual number of atoms in a single molecule.
Molecular Formulas
Molecular representations, on the other hand, show the exact number of atoms of each element in a molecule of the substance. Using the glucose example again, its molecular formula is C6H12O6, providing a precise count of the atoms. This formula reflects the actual structure and size of the compound.
While the empirical formula is often used for simplicity, the molecular formula is essential when exact information is needed for applications like chemical reactions or understanding physical properties.
Understanding Coefficients in Chemical Equations
In reactions, the numbers placed before the compounds indicate the proportions in which substances combine or are produced. These numbers, known as coefficients, play a vital role in balancing reactions and ensuring accurate calculations.
Role of Coefficients in Reactions
Coefficients provide the necessary information to understand how many molecules or moles of each substance are involved in a reaction. They help determine the relative amounts of reactants and products, ensuring that the law of conservation of mass is followed.
How Coefficients Impact Reactions
Properly assigning coefficients in an equation is essential for balancing, ensuring the number of atoms of each element remains the same on both sides. This process is crucial for predicting reaction outcomes and scaling reactions for practical use.
- In unbalanced reactions, coefficients may be adjusted to achieve a balanced equation.
- They help calculate molar relationships between substances involved in the reaction.
- Accurate coefficients are required for quantitative analysis, such as determining yields or reaction rates.
Balancing Chemical Equations Made Simple
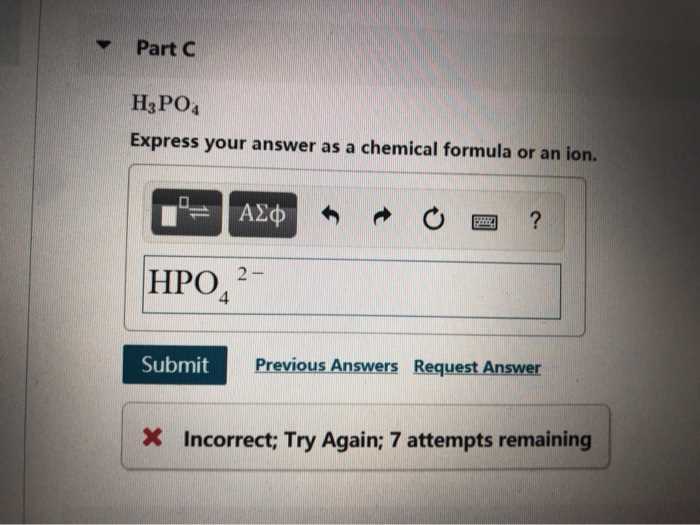
Ensuring that an equation reflects the same number of atoms on both sides is a crucial step in understanding reactions. This process is essential for accurately representing how substances interact and transform during a reaction.
To achieve balance, it is important to adjust the quantities of each substance so that the law of conservation of mass holds true. This involves manipulating the numbers in front of each compound, called coefficients, to ensure that the number of atoms for each element is the same on both sides of the equation.
Step-by-Step Process
The process of balancing starts with counting the number of atoms of each element in the reactants and products. Once this is done, you can begin adjusting coefficients to achieve the correct balance. Here is a simplified step-by-step guide:
| Step | Action |
|---|---|
| 1 | Write down the unbalanced equation. |
| 2 | Count the atoms of each element on both sides of the equation. |
| 3 | Adjust coefficients to balance one element at a time, starting with the most complex compound. |
| 4 | Recheck the balance of atoms after each adjustment. |
| 5 | Ensure all coefficients are in the smallest whole number ratio. |
With practice, this process becomes more intuitive, allowing for easier manipulation and a better understanding of the interactions in a given reaction.
How to Memorize Chemical Symbols Effectively
Memorizing the abbreviations used for elements in scientific contexts can be challenging, but with the right strategies, it becomes much easier. Understanding the patterns and using different memorization techniques can significantly enhance your ability to recall these symbols quickly.
Utilize Mnemonics

One of the most effective ways to remember the abbreviations is by creating mnemonics. These are memory aids that link the symbol to a word or phrase. For example, for “Na” (Sodium), a mnemonic like “Not A” could help remind you of its symbol. This method turns abstract symbols into something more familiar and easier to recall.
Practice with Flashcards
Using flashcards is another highly effective technique. Write the element’s name on one side and the abbreviation on the other. Reviewing these cards regularly helps reinforce memory. You can also use digital flashcard apps that allow for spaced repetition, further aiding in memorization over time.
Repetition and association are key when learning new information. By actively engaging with the material and using different methods to link the symbols to memorable cues, you can make the process of memorizing much more efficient.
Practical Uses of Chemical Formulas in Science
The use of symbolic representations in science plays a critical role in simplifying complex information and enabling quick communication of key concepts. These representations are utilized across various scientific fields to understand and predict reactions, substances, and processes. By expressing the composition of substances, researchers can make informed decisions and calculations.
Applications in Medicine
In the field of medicine, symbolic representations help identify the composition of drugs and their interactions with the human body. They are essential for:
- Creating accurate dosage forms.
- Understanding drug metabolism.
- Predicting side effects based on molecular structure.
Applications in Environmental Science
Environmental scientists rely on these symbolic representations to assess pollutants and their impact on ecosystems. Some uses include:
- Tracking the spread of contaminants in water and soil.
- Evaluating chemical cycles in nature, such as carbon or nitrogen cycles.
- Developing sustainable solutions for waste management.
By employing these symbolic notations, scientists can work more efficiently, ensuring accuracy and clarity in their findings across diverse fields of study.
Learning the Basics of Ionic Compounds
Understanding the composition and properties of substances formed through the transfer of electrons is a foundational concept in science. These compounds are formed when atoms of different elements join together through attractive forces that result from the loss or gain of electrons. The relationship between these particles and their ability to form stable structures plays a key role in numerous chemical processes and reactions.
At the core of these substances lies the combination of positively charged and negatively charged particles, which balance each other to create stable configurations. The attraction between these oppositely charged ions is strong, allowing these compounds to exhibit unique properties such as high melting points, electrical conductivity in molten or dissolved forms, and solubility in water.
As you explore this area of study, understanding the nature of these compounds will help in explaining how certain materials interact with each other and how they are used in real-world applications such as in electronics, medicine, and industrial processes.
Tips for Writing Complex Organic Formulas
Constructing detailed representations of organic compounds requires attention to the arrangement of atoms and bonds. These structures are often intricate, involving carbon chains, functional groups, and various types of bonds, each contributing to the compound’s overall characteristics. Mastering the writing of these structures helps in identifying their properties and predicting their behavior in reactions.
Here are some essential tips to guide you in writing complex organic structures accurately:
- Start with the backbone: Begin by identifying the longest carbon chain and the main structure. This serves as the backbone to which other groups will be attached.
- Use appropriate notation for bonds: Ensure that single, double, or triple bonds are clearly represented between carbon atoms. If no bond is drawn, it is assumed to be a single bond.
- Include functional groups: Pay attention to the specific groups attached to the carbon chain, such as alcohols, ketones, or amines. These groups are often crucial in determining the compound’s chemical reactivity.
- Apply correct stereochemistry: In cases where molecules exhibit stereoisomerism, use proper notation for the spatial arrangement of atoms, especially in cyclic compounds or molecules with double bonds.
- Verify atom counts: Always double-check the number of atoms in the structure to ensure they match the compound’s molecular composition.
By following these tips, you will be able to effectively write and interpret the structures of organic compounds, making it easier to understand their reactions and applications in various fields such as pharmacology and materials science.
Using Chemical Formulas in Problem Solving
The ability to read and manipulate molecular representations is essential in tackling a variety of scientific challenges. By understanding the composition of substances and their interactions, one can approach problems involving stoichiometry, reaction mechanisms, and material analysis. These representations serve as a tool to quantify and predict outcomes in both experimental and theoretical scenarios.
Understanding the Role of Subscripts and Coefficients
In problem-solving, the numbers within the molecular representations provide valuable information about the ratios of elements or molecules involved in a reaction. Subscripts indicate the number of atoms of each element in a compound, while coefficients help balance the equation to satisfy the law of conservation of mass. Being able to interpret and use these numbers correctly is crucial for making accurate calculations.
Applying Representations in Real-Life Scenarios
Whether calculating the amount of reactants needed for a reaction or determining the yield of a product, molecular representations help simplify these complex tasks. For example, when working with moles, knowing how to convert between the amount of a substance and the number of molecules or atoms is key to solving problems in chemistry and related fields. This application is vital in various industries, from pharmaceuticals to environmental science.