
In various fields, handling large or small values efficiently is crucial. To make complex numbers more manageable, a system exists that helps represent them in a more concise way. This approach not only saves space but also enhances accuracy when performing mathematical operations.
When dealing with vast quantities or tiny measurements, it’s easy to get overwhelmed by the sheer size of the numbers. By transforming them into a more compact form, it becomes possible to carry out calculations without the confusion that often arises from excessively long digits. This method is used across many disciplines, from engineering to physics, due to its practical benefits.
Understanding how to manipulate numbers in this form is key to performing advanced calculations with confidence. Whether multiplying, dividing, or performing other operations, this technique offers a streamlined approach that simplifies even the most complex equations.
How to Express Numbers in Scientific Form
Representing numbers in a more compact and manageable way is essential for handling extremely large or small values. This method helps reduce the complexity of calculations and makes working with such numbers more straightforward. Whether you’re dealing with measurements in science, engineering, or finance, transforming values into this format is a valuable skill.
To convert a number into this condensed format, consider the following basic steps:
- Identify the significant digits of the number, focusing on the non-zero values.
- Place the decimal point after the first non-zero digit.
- Count the number of places the decimal point has moved and express this as an exponent of ten.
- Adjust the number accordingly based on whether it’s a large or small value.
For example, to express 6,000,000 in a more compact form:
- The significant digits are 6.
- The decimal moves 6 places to the left.
- The result is 6 × 106.
When dealing with smaller numbers, such as 0.00045, the process is similar:
- The significant digits are 45.
- The decimal moves 4 places to the right.
- The result is 4.5 × 10-4.
By following these steps, large and small values become much easier to handle, enabling smoother and more efficient mathematical operations across various fields.
Understanding the Basics of Scientific Notation
In many fields, working with extremely large or small numbers can be cumbersome. To make calculations easier and more efficient, a method exists that allows these numbers to be represented in a more compact form. This approach not only reduces the complexity of numbers but also makes them easier to work with, especially when performing mathematical operations like multiplication, division, or addition.
The core idea behind this method is to express a number as the product of a base and an exponent. The base is typically a number between 1 and 10, while the exponent indicates how many times the base should be multiplied or divided by 10. This helps represent both very large and very small values in a consistent and manageable way.
For instance, the number 1,000,000 can be written as 1 × 106, and the number 0.00045 can be written as 4.5 × 10-4. In both cases, the base (1 and 4.5) is adjusted, and the exponent tells us how many times the base should be scaled up or down using powers of 10. Understanding this system makes it easier to handle complex numbers in various mathematical and scientific contexts.
Why Use Scientific Notation for Large Numbers
When dealing with very large quantities, the numbers can quickly become unwieldy and difficult to manage. By transforming these values into a more concise form, it becomes much easier to perform operations and communicate results. This method reduces the complexity of working with large figures, making calculations more straightforward and less prone to error.
One of the main reasons for using this system is that it allows numbers to be expressed in a way that is both efficient and easy to manipulate. Instead of writing out long strings of zeros, the value is condensed into a small base number multiplied by a power of ten. This drastically reduces the space required to write or store such values, making them more practical in both written and digital formats.
For example, expressing the number 1,000,000,000 as 1 × 109 not only saves space but also makes it easier to compare numbers of vastly different magnitudes. This streamlined approach is especially useful in fields such as physics, engineering, and computer science, where large quantities are common and precision is essential.
Converting Regular Numbers to Scientific Notation
Transforming standard numbers into a more compact form is essential for managing large and small values more efficiently. The process involves adjusting the number so that it is represented as a product of a base and an exponent. This allows the number to be expressed more succinctly, making mathematical operations simpler and quicker to perform.
Here are the basic steps to convert a number:
- Identify the significant digits – Focus on the meaningful figures of the number, leaving out any zeros that do not contribute to its value.
- Place the decimal point – Move it so that it follows the first non-zero digit, making the base number between 1 and 10.
- Count the movement – Determine how many places the decimal point was shifted. This count becomes the exponent of 10.
- Write the result – Combine the base with the power of 10 to create the final expression.
For example, to convert 25,000,000 into this form:
- The significant digits are 25.
- The decimal moves 7 places to the left.
- The result is 2.5 × 107.
For smaller numbers, such as 0.00032:
- The significant digits are 32.
- The decimal moves 4 places to the right.
- The result is 3.2 × 10-4.
By following these steps, any regular number can be efficiently transformed into a more manageable format, making it easier to work with in calculations.
Key Rules for Scientific Notation Simplification
When working with large or small values, understanding the key principles of transforming and manipulating them is essential for accurate and efficient calculations. These rules ensure consistency and clarity, enabling smooth operations like multiplication, division, and addition of numbers in their compact forms.
Understanding Powers of Ten
The exponent plays a crucial role in representing the magnitude of a number. When converting or adjusting numbers, it’s important to carefully track how the decimal moves and to adjust the power of ten accordingly. For positive exponents, the decimal moves to the right, indicating larger values, while negative exponents move the decimal to the left, representing smaller values. The exponent must always reflect the total number of places the decimal has been shifted.
Managing Significant Figures
It’s vital to focus on the significant figures when expressing numbers. These are the digits that carry meaningful information about the precision of the number. Zeros that are used solely for place value should not be included as part of the significant digits. Properly identifying and handling these figures ensures the accuracy of the final result.
By adhering to these rules, the process of converting and operating on numbers in compact form becomes more straightforward, reducing the potential for errors in calculations.
Scientific Notation and Its Applications in Science
The use of compact forms for large and small values is essential in various scientific fields. This method allows scientists to represent numbers that would otherwise be too cumbersome to handle. In areas such as physics, chemistry, and astronomy, where measurements can span enormous ranges, this approach simplifies calculations and communication of results.
Applications in Physics
In physics, measurements of distances, forces, and energy often involve numbers that are either extremely large or small. By expressing these quantities in a compact form, scientists can perform calculations more easily and avoid errors caused by the scale of the values. For instance, the mass of subatomic particles is incredibly small, while the distances between stars are vast. Using a more efficient way to express these values is crucial in everyday scientific practice.
Applications in Chemistry and Astronomy
In chemistry, the scale of molecular interactions and concentrations requires a method to handle both very small and very large numbers. Similarly, in astronomy, the size of the universe and the age of celestial bodies necessitate the use of condensed numerical representations. These applications benefit greatly from the ability to express values clearly and without unnecessary complexity.
| Field | Example | Expression |
|---|---|---|
| Physics | Speed of light | 3.00 × 108 m/s |
| Chemistry | Avogadro’s number | 6.022 × 1023 mol-1 |
| Astronomy | Distance to the nearest star | 4.24 × 1013 km |
By adopting this method, scientists in these fields can manage and manipulate data with greater precision and efficiency, ultimately leading to more accurate conclusions and advancements in research.
Common Mistakes in Writing Scientific Notation
When converting large or small numbers into their compact forms, several common errors can arise. These mistakes typically occur during the adjustment of the decimal point or the handling of significant figures. Recognizing and avoiding these errors is essential to ensuring the accuracy and clarity of the final result.
Misplacing the Decimal Point
One of the most frequent mistakes is incorrectly moving the decimal point. When transitioning a number into a more condensed format, the decimal should be positioned immediately after the first non-zero digit. If the decimal is placed too far to the left or right, the power of ten will be incorrect, leading to inaccurate results.
Incorrect Handling of Exponents
Another common mistake is miscalculating the exponent. The exponent reflects how many times the decimal point has been shifted. It’s important to ensure the direction of the shift is correct–moving the decimal to the right for large numbers results in a positive exponent, while shifting to the left for smaller numbers gives a negative exponent. Mistakes in determining this shift often result in an incorrect representation of the number.
By being mindful of these common errors, the process of expressing numbers in a more compact form can be done with greater accuracy, avoiding confusion and ensuring correct calculations.
How to Handle Negative Exponents Effectively
Negative exponents are used to represent very small numbers, and understanding how to manage them is crucial when expressing quantities in a condensed form. The key to handling these exponents effectively is recognizing that a negative exponent indicates the reciprocal of the number raised to the corresponding positive power. This allows for a straightforward transformation between large and small values.
To work with negative exponents, the main principle is to move the decimal point to the left. For example, in the number 3.2 × 10-4, the exponent tells you to shift the decimal point four places to the left, resulting in 0.00032. This method is especially useful when dealing with measurements in fields such as chemistry and physics, where values like concentrations or atomic radii often fall within small ranges.
It’s important to note that the same rules for positive exponents apply, but with a shift towards smaller values. Practicing with negative exponents will help ensure accuracy when converting or calculating numbers in different scientific contexts.
Understanding Significant Figures in Scientific Notation
When working with large or small numbers, it is crucial to focus on the meaningful digits that convey the precision of a value. These digits are known as significant figures, and they play a key role in ensuring that the expressed numbers accurately represent the precision of measurements or calculations. Recognizing and correctly identifying these figures is essential for maintaining the integrity of scientific data.
The significant figures in a number include all non-zero digits, any zeros between significant digits, and any trailing zeros in a decimal number. However, leading zeros (zeros before the first non-zero digit) are not counted as significant. For example, in the number 0.00456, the significant figures are 4, 5, and 6. This principle helps clarify the accuracy of the measurements and ensures consistency across scientific calculations.
When expressing values in a condensed form, it’s important to preserve the same level of precision. Whether you are converting large numbers or handling small ones, correctly identifying and preserving the significant figures ensures that the results are both accurate and meaningful.
Practical Examples of Scientific Notation Usage

The ability to express numbers in a condensed form is essential in many real-world scenarios. In fields like physics, chemistry, and engineering, this method is frequently employed to handle values that are either extremely large or small, making calculations and comparisons more manageable. Below are some practical examples where this approach is commonly used.
One of the most common applications is in astronomy, where the vast distances between celestial bodies are measured. For instance, the distance from Earth to the nearest star, Proxima Centauri, is about 4.24 × 1013 kilometers. Such large numbers would be cumbersome to write out in full, so expressing them in a more compact form is not only convenient but necessary for clarity.
In chemistry, this method is used to express Avogadro’s number, which represents the number of particles in a mole. The value is approximately 6.022 × 1023 particles per mole, a figure so large that expressing it in full would be impractical for calculations and communication.
Another example is in electronics, where the size of components like capacitors and resistors is often given in small units. For example, a capacitor might have a capacitance of 4.7 × 10-6 farads, which represents a very small quantity. Using this form makes it easier to perform calculations without having to work with long decimal numbers.
These examples illustrate the practical advantages of expressing numbers in a more compact, manageable form, allowing for easier calculations and clearer communication across various fields of study and industry.
Scientific Notation in Different Fields of Study
In many academic disciplines, the ability to express values in a more compact form is essential for simplifying complex calculations and improving clarity. This method of representing numbers plays a critical role in various fields, from the vastness of space exploration to the microscopic scale of molecular biology. Each field uses this technique to handle extreme values, whether large or small, to enhance precision and streamline problem-solving processes.
In physics, for example, the size and scale of the universe often require the use of this form. Distances between planets and stars, the mass of subatomic particles, and the energy involved in atomic reactions are typically expressed in this way to make the numbers more manageable. A measurement like the speed of light, 3 × 108 meters per second, is far more practical to work with in its condensed form than as a full number.
In biology and chemistry, this form is widely used when discussing quantities at the molecular or atomic level. The number of molecules in a mole, or the size of cells and viruses, can be represented compactly to facilitate calculations and comparisons. For instance, the mass of an individual atom is often written as 1.66 × 10-27 kilograms, making it easier to compare with other masses in experiments.
Similarly, in economics, large financial figures, such as national debt or the GDP of large economies, are often presented in a more manageable form. This allows economists to perform large-scale calculations more efficiently, while also aiding in the communication of complex data to the public in a more digestible format.
These examples highlight the widespread use of this method across different disciplines, illustrating its importance in simplifying calculations and enhancing understanding across a range of scientific and practical applications.
How to Multiply Numbers in Scientific Notation
When multiplying numbers expressed in a compact form, the process is relatively straightforward once you understand the basic rules. The key steps involve multiplying the coefficients (the numbers in front) and then applying the laws of exponents to the powers of 10. This method ensures that the final result is also expressed concisely and accurately.
Step 1: Multiply the Coefficients
Start by multiplying the numbers in front of the powers of 10, also known as the mantissas or coefficients. For example, when multiplying 3 × 104 by 2 × 103, you first multiply the 3 and 2 to get 6.
Step 2: Add the Exponents
Next, add the exponents of the two numbers. For instance, in the previous example, the exponents 4 and 3 are added together to get 7. Therefore, the product of the two numbers becomes 6 × 107.
By following these simple steps, you can easily multiply numbers expressed in this form and maintain the precision of your calculations. This method applies to any pair of numbers and is fundamental for working with large or small quantities in various fields of study.
Division of Numbers Using Scientific Notation
Dividing numbers expressed in a compact form is a simple yet powerful method for handling large and small values efficiently. The process involves dividing the coefficients and subtracting the exponents, which allows for a clear and accurate result. This technique is commonly used in various scientific and mathematical calculations to streamline complex operations.
Step 1: Divide the Coefficients
To begin, divide the numerical values in front of the powers of 10. For example, if you have 6 × 105 divided by 3 × 102, you first divide 6 by 3 to get 2.
Step 2: Subtract the Exponents
Next, subtract the exponent of the denominator from the exponent of the numerator. In this example, you subtract 2 from 5, resulting in an exponent of 3. Thus, the division of the two numbers becomes 2 × 103.
| Expression | Step 1: Divide Coefficients | Step 2: Subtract Exponents | Result |
|---|---|---|---|
| 6 × 105 ÷ 3 × 102 | 6 ÷ 3 = 2 | 5 – 2 = 3 | 2 × 103 |
By following these steps, you can easily divide numbers in this form, keeping your calculations both manageable and precise. This technique is especially useful in fields like physics, chemistry, and engineering, where very large or very small values are commonly encountered.
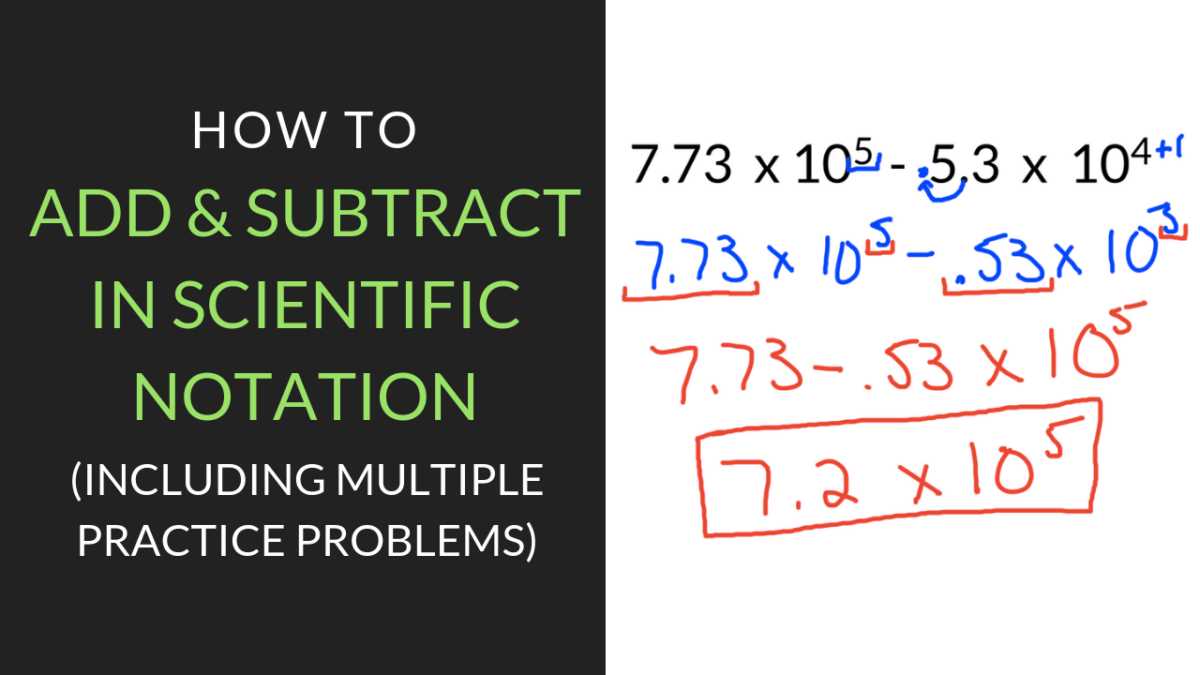
Adding and Subtracting with Scientific Notation
When performing addition or subtraction with numbers in compact form, it is crucial to ensure that the exponents are the same before proceeding with the operation. This approach guarantees that the numbers are on the same scale and allows for accurate calculations. After adjusting the exponents, the operation can be carried out as usual by adding or subtracting the coefficients.
Before you begin adding or subtracting, the first step is to ensure that both numbers have the same exponent. If the exponents are different, one of the numbers must be adjusted by shifting its decimal point to match the other. Once the exponents are aligned, you can add or subtract the numbers as you would with regular values.
| Expression | Step 1: Align Exponents | Step 2: Add/Subtract Coefficients | Result |
|---|---|---|---|
| 5 × 103 + 2 × 104 | Convert 5 × 103 to 0.5 × 104 | 0.5 + 2 = 2.5 | 2.5 × 104 |
| 8 × 106 – 3 × 106 | Exponents are the same | 8 – 3 = 5 | 5 × 106 |
By following these steps, adding and subtracting numbers in this form becomes straightforward. This technique is useful in many fields such as engineering, astronomy, and computer science, where handling both large and small numbers efficiently is essential.
The Role of Powers of Ten in Notation
The powers of ten are fundamental in representing values efficiently, especially when dealing with extremely large or small numbers. These powers allow us to express numbers in a way that makes them more manageable and easier to work with. By using exponents, we can scale numbers up or down in a standardized manner, which is essential in many fields such as physics, engineering, and computer science.
When a number is expressed with powers of ten, the exponent indicates how many places the decimal point needs to be moved. This method simplifies calculations and allows us to focus on the significant figures of the number, while the exponent takes care of the scale.
- Positive Exponents: Used for large numbers. A positive exponent indicates that the decimal point has been moved to the right, increasing the value.
- Negative Exponents: Used for small numbers. A negative exponent shows that the decimal point has been moved to the left, reducing the value.
- Standardization: The power of ten provides a consistent way to represent numbers across different magnitudes, making comparisons and calculations easier.
By understanding and utilizing the role of powers of ten, we can manipulate and interpret numbers in a more efficient manner, which is especially useful when working with measurements in scientific and technical contexts.
Advantages of Using Scientific Notation in Calculations
Utilizing this method of expression in calculations offers numerous benefits, particularly when working with very large or very small numbers. It provides a compact and efficient way to handle values that would otherwise be unwieldy or difficult to manage. Instead of writing out long strings of zeros, numbers are simplified, allowing for easier computation and clearer understanding.
Efficiency in Computation
When dealing with calculations involving vast or minuscule quantities, converting numbers into this format helps speed up mathematical operations. Multiplication and division are straightforward, as the powers of ten can be combined, and calculations are less prone to error.
Improved Readability
By using a standardized format, long numbers are condensed, making them easier to read and interpret. This is especially helpful in scientific and engineering disciplines where clarity is crucial.
- Compact Representation: Reduces the space needed to display numbers, making them more manageable.
- Accuracy in Large-Scale Calculations: Keeps numbers precise, especially when working with measurements like distances in astronomy or molecular quantities.
- Standardization Across Fields: Provides a consistent approach for handling numbers in various scientific fields, ensuring uniformity in data presentation.
In summary, using this format streamlines the process of computation, improves clarity, and reduces the potential for errors, making it an essential tool for professionals working with large datasets or complex equations.