In science, measuring substances at a microscopic level can be a challenging task. To solve this problem, a standard unit has been developed, allowing scientists to quantify and compare particles in a consistent way. This method plays a vital role in chemical reactions, where precise amounts of elements and compounds need to be combined.
One key unit of measurement provides a bridge between the macroscopic world we see and the microscopic realm of atoms and molecules. By using this unit, chemists can perform complex calculations and understand how different substances interact on a molecular level. It is essential for anyone looking to explore the science behind chemical processes and reactions.
Understanding how this unit works and how it is applied allows for a deeper appreciation of chemical formulas, stoichiometry, and even gas laws. Its importance is not limited to theoretical chemistry but extends to practical applications in industries, research, and even everyday life.
The Mole as a Measurement of Matter
In scientific studies, quantifying substances on a microscopic scale presents a unique challenge. To facilitate understanding, a standard unit has been created that helps in determining the number of particles within a given amount of material. This concept is crucial in chemistry, allowing for accurate calculations in various fields, from chemical reactions to molecular structures.
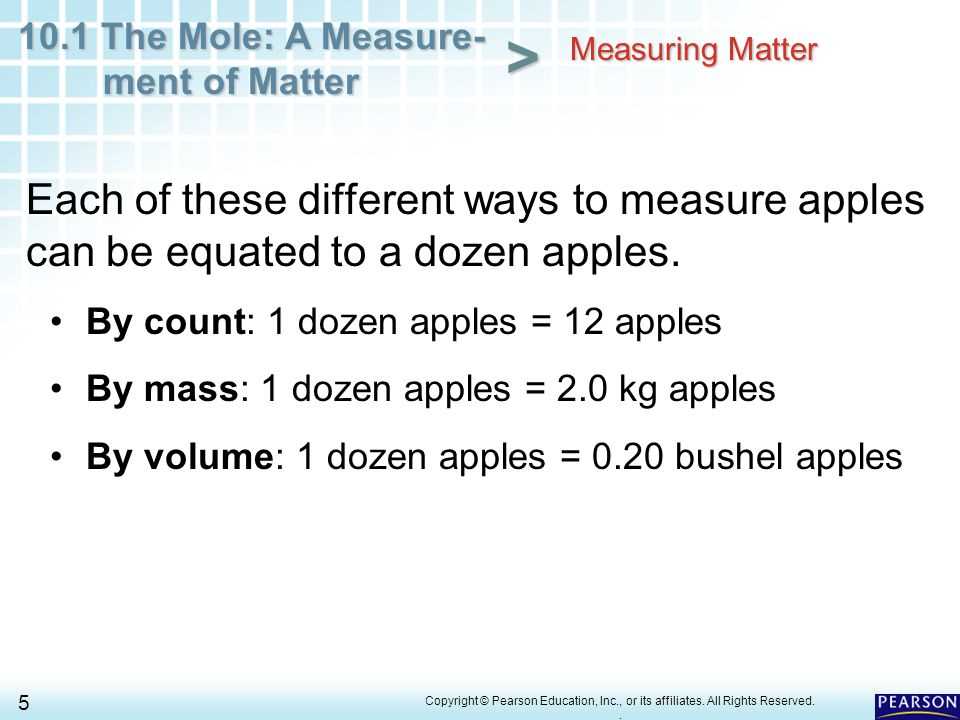
Role in Chemical Reactions
This unit serves as a bridge between the atomic world and practical chemistry. When performing chemical reactions, knowing how much of each substance is involved is essential for predicting outcomes and ensuring proper ratios. Without this unit, scientists would struggle to measure and balance the components of reactions effectively.
- Enables accurate stoichiometric calculations
- Helps determine the amount of reactants and products
- Essential for understanding reaction yields
Applications in Molecular Science
Beyond chemical reactions, this unit is indispensable in the study of molecular structures. By using it, chemists can calculate molecular weights, determine concentrations in solutions, and analyze the behavior of gases under various conditions. It is foundational to fields like biochemistry, environmental science, and materials science.
- Determining molecular weight in compounds
- Calculating concentrations in solutions
- Analyzing gas laws and behavior
Understanding the Concept of a Mole
In scientific fields, particularly chemistry, it’s essential to find ways to describe quantities of substances that exist at a microscopic scale. This concept allows scientists to work with numbers that are practical for laboratory settings, where atoms and molecules are the fundamental units of matter. The idea behind this concept is to provide a standard unit for counting these incredibly small entities, much like how we use units such as grams or liters for larger amounts of material.
Fundamental Ideas Behind This Unit
To grasp the significance of this concept, it’s important to understand its role in bridging the gap between the atomic scale and real-world applications. Without it, it would be nearly impossible to quantify and compare substances at the atomic level, as the numbers involved would be extraordinarily large and cumbersome.
- Represents a fixed quantity of particles
- Links macroscopic measurements to microscopic entities
- Allows for consistency in chemical calculations
Historical Development and Usage
Over time, the idea has evolved from theoretical models to practical applications, becoming a cornerstone of modern chemistry. It provides a way to quantify atoms, molecules, and ions in a manageable way, making it easier to conduct experiments and interpret results. This unit’s definition is rooted in Avogadro’s number, a fundamental constant in chemistry.
- Originated from Avogadro’s insights
- Defines a specific number of particles
- Used universally in chemical studies
How Moles Relate to Avogadro’s Number
In scientific studies, particularly chemistry, it’s crucial to have a system that connects the microscopic world to something tangible. To quantify the tiny particles that make up substances, a standard quantity is used. This quantity is linked directly to a specific number, known as a fundamental constant, which serves as the cornerstone for working with quantities of particles in a meaningful way.
Avogadro’s number plays an essential role in this system. It defines the exact number of particles that correspond to one unit, allowing scientists to convert between the atomic and macroscopic scales. This number provides a constant reference point, ensuring consistency in calculations and experiments, regardless of the substance being studied.
- Defines a specific count of atoms or molecules
- Allows conversion between atomic and macroscopic amounts
- Enables accurate chemical calculations
Avogadro’s number is universally applied in chemistry, linking a numerical value with a specific quantity of particles, making it possible to measure and manipulate substances in a practical way. It is the foundation of many calculations involving substances at the atomic level, providing clarity and precision for scientific studies.
The Role of Moles in Chemistry
In chemistry, understanding the quantities and relationships between substances is fundamental to conducting accurate experiments and reactions. A system has been developed that allows scientists to count and compare particles at the atomic level, making it easier to manipulate chemical equations and study reactions. This system is essential in translating the microscopic world of atoms and molecules into practical, real-world applications.
By using a standardized quantity, chemists are able to measure and predict the behavior of substances with precision. This unit plays a crucial role in determining reaction yields, calculating concentrations, and understanding molecular structures. Without it, the study of chemistry would be inefficient and cumbersome, as direct measurements of individual particles would be impractical.
- Facilitates accurate stoichiometric calculations
- Enables the prediction of reaction outcomes
- Essential for determining concentrations and molecular structures
Furthermore, this system allows for consistency across scientific research, ensuring that experiments can be replicated and results compared regardless of the specific substances being studied. It serves as a foundation for various fields of chemistry, from organic chemistry to biochemistry and materials science.
Why Moles Are Essential in Chemical Reactions

In chemical reactions, the precise combination of substances is crucial for achieving the desired results. To effectively predict and control these reactions, scientists rely on a standardized unit that allows them to calculate the exact amounts of each substance involved. This concept is key to balancing reactions, determining reaction yields, and ensuring that all reactants are fully utilized.
Using this unit, chemists can relate the quantities of reactants to products, ensuring that reactions proceed in the right proportions. Without this system, it would be nearly impossible to accurately scale reactions or apply them in practical settings, such as industrial manufacturing or laboratory experiments.
| Substance | Required Quantity | Reaction Outcome |
|---|---|---|
| Hydrogen | 2 moles | 1 mole of water |
| Oxygen | 1 mole | 1 mole of water |
This unit helps ensure that chemical reactions are balanced, providing a way to calculate the correct proportions of reactants. It also simplifies the prediction of product amounts and reaction times, which is crucial for optimizing industrial and laboratory processes.
Defining the Mole in Simple Terms
In chemistry, understanding how to count extremely small particles, like atoms and molecules, is essential for working with substances. While it’s difficult to directly count such tiny entities, scientists have created a system that helps translate atomic numbers into manageable quantities. This system allows for more straightforward calculations and practical application in experiments.
Understanding the Basic Concept

At its core, this unit represents a specific quantity of particles, making it easier to work with substances in the lab. Think of it like how a dozen eggs represents twelve individual eggs. Similarly, this unit groups a vast number of atoms or molecules into one manageable “count,” so scientists can use it for reactions and calculations.
Why It Matters in Chemistry
Without this system, dealing with the quantities involved in chemical reactions would be impossible. Instead of having to work with billions of atoms at a time, this concept allows chemists to scale reactions up or down, using quantities that are practical for experiments and industry.
The Significance of the Mole in Science
In scientific research, having a standard way to count and quantify particles at the atomic and molecular level is essential for accurate calculations and consistent results. This system allows chemists to work with substances in a way that connects the microscopic world to macroscopic measurements. Its importance extends beyond chemistry, influencing various fields of science and engineering.
Key Role in Chemical Studies
This unit is fundamental to the study of chemical reactions and the behavior of different substances. By providing a consistent way to measure atoms, molecules, and ions, it enables scientists to conduct precise experiments and make predictions about how substances will react with each other. Without this system, predicting reaction yields and calculating chemical concentrations would be nearly impossible.
- Essential for reaction balancing and stoichiometry
- Facilitates precise concentration calculations in solutions
- Helps understand molecular behavior and properties
Impact on Other Scientific Fields
Beyond chemistry, this concept also plays a critical role in fields such as physics, biology, and environmental science. It allows scientists to measure and quantify interactions at the atomic and molecular levels, which is crucial for understanding everything from molecular biology to materials science.
- Influences biochemistry and molecular biology research
- Essential in environmental studies for pollutant analysis
- Supports the development of new materials and technologies
Unit Conversions Involving the Mole
In scientific work, it’s often necessary to convert between different units when dealing with quantities of particles. This allows scientists to translate atomic and molecular scales into more practical measurements. These conversions are crucial when working with various substances, ensuring that calculations remain consistent and accurate across different units of mass, volume, and quantity.
When converting between these units, it’s essential to use appropriate conversion factors. For example, one can convert from grams to atoms or molecules by knowing the molecular weight of a substance and its corresponding quantity of particles. This process helps scientists understand how much of a substance is involved in a reaction or how much is needed for an experiment.
- Mass to particles: Use molecular weight and Avogadro’s number
- Volume to particles: Use molar volume at specific conditions (e.g., temperature and pressure)
- Particles to mass: Apply atomic mass units and conversion factors
Example: To convert the mass of a substance into the number of particles, you would first find the molecular weight, then use Avogadro’s number to determine how many individual particles are present in a given amount of the substance.
Calculating Moles in Real-World Examples
In practical applications, it is often necessary to calculate the amount of substance required for a specific reaction or to understand the composition of a material. These calculations are based on the relationship between mass and the number of particles present. Real-world examples show how these calculations can be applied to various scenarios, from chemical reactions in laboratories to industrial processes.
Example 1: Chemical Reaction in a Lab
Suppose you are working with a chemical reaction that requires a certain amount of reactant to produce a desired product. By knowing the molar mass of the reactant and its chemical formula, you can calculate how much of the substance is needed to achieve the reaction’s optimal outcome.
Example: If you need 5 grams of sodium chloride (NaCl) for a reaction, the molar mass of sodium chloride is 58.44 g/mol. Using the formula:
Number of moles = mass / molar mass
Number of moles of NaCl = 5 g / 58.44 g/mol = 0.0855 moles
This calculation tells you how many moles of NaCl are needed for the reaction to proceed efficiently.
Example 2: Determining Concentration in Solutions
In laboratory settings, it’s often important to know the concentration of a substance in a solution. By calculating the amount of substance in terms of its molar quantity, you can determine the molarity of the solution. This is especially useful in titrations and other analytical techniques.
Example: To prepare 1 liter of a 0.5 M solution of sodium hydroxide (NaOH), you need to calculate how many grams of NaOH are required:
0.5 M = 0.5 moles per liter
For 1 liter, you need 0.5 moles of NaOH. The molar mass of NaOH is 40.00 g/mol, so:
Mass of NaOH = 0.5 moles × 40.00 g/mol = 20.00 grams
This calculation tells you how much NaOH to weigh out to make the desired solution.
The Relationship Between Moles and Mass
Understanding how quantities of substances are related to their mass is a fundamental concept in chemistry. This relationship allows scientists to calculate how much of a substance is needed for a reaction or experiment, and it also helps them translate between the weight of a substance and the number of particles it contains. The connection between mass and quantity is based on the molecular weight of a substance and its conversion to practical quantities used in labs and industries.
To calculate the amount of substance in terms of moles from its mass, you need to know the substance’s molar mass, which is the mass of one mole of particles. The formula to convert between mass and moles is:
Number of moles = mass of substance (g) / molar mass (g/mol)
| Substance | Molar Mass (g/mol) | Mass (g) | Number of Moles |
|---|---|---|---|
| Water (H₂O) | 18.02 | 36.04 | 2.0 |
| Sodium Chloride (NaCl) | 58.44 | 58.44 | 1.0 |
| Carbon Dioxide (CO₂) | 44.01 | 88.02 | 2.0 |
In the table above, we can see how different substances are related to their mass in terms of the number of moles. By using the molar mass, scientists can easily calculate the number of moles present in any given mass of a substance.
How Moles Impact Chemical Formulas
In chemistry, understanding how quantities of elements relate to each other in a compound is essential for forming accurate chemical formulas. This relationship plays a significant role in determining the composition of substances and predicting how they behave during reactions. The number of particles involved in a compound influences its molecular structure and can impact various properties such as reactivity and stability.
Elemental Proportions in Compounds
When chemists write a formula for a compound, the subscripts in the formula represent the ratio of elements in terms of their atomic quantities. This ratio is directly tied to the amount of each element present in a given sample of the compound. For instance, the formula for water (H₂O) shows that each molecule contains two atoms of hydrogen for every one atom of oxygen, which can be translated into molar quantities to guide reactions.
- Example 1: In carbon dioxide (CO₂), the formula indicates there are two atoms of oxygen for every one atom of carbon. This ratio helps chemists calculate the exact number of atoms in any given mass of CO₂.
- Example 2: In sodium chloride (NaCl), the formula reveals that one sodium atom bonds with one chloride atom, forming a 1:1 ratio that is essential for understanding its behavior in different environments.
Calculations Based on Chemical Formulas
By knowing the molar mass of each element involved in a compound, scientists can calculate the number of moles present in any sample. This allows them to determine the exact amount of substance needed to react with others in a chemical equation. For example, in a reaction where sodium reacts with chlorine to form sodium chloride, understanding the number of moles helps chemists ensure the correct proportions of reactants are used for complete reaction efficiency.
The Mole and its Use in Stoichiometry
Stoichiometry is the branch of chemistry that focuses on the quantitative relationships between reactants and products in chemical reactions. Understanding how much of each substance is involved in a reaction is critical for predicting outcomes and ensuring that reactions proceed efficiently. One of the most essential tools in stoichiometry is a system for relating the amounts of substances involved, helping scientists to calculate the precise proportions required for any given reaction.
Application in Reaction Calculations
In chemical reactions, reactants combine in fixed proportions to produce products. Using a system to quantify these substances, chemists can calculate how much of each reactant is needed or how much product will be produced. By translating the amounts of substances into measurable units, the process becomes manageable and precise.
- Example 1: In a reaction between hydrogen and oxygen to form water, stoichiometry allows scientists to calculate the exact amount of hydrogen needed to react with a specific amount of oxygen.
- Example 2: For reactions in industrial processes, such as the production of ammonia, stoichiometric calculations ensure that raw materials are used efficiently, minimizing waste and maximizing output.
Step-by-Step Stoichiometric Calculations
Stoichiometric calculations typically follow these steps:
- Write a balanced chemical equation for the reaction.
- Convert known quantities of substances to moles.
- Use the mole ratios from the balanced equation to calculate the required amount of another substance.
- Convert moles back to the desired units (e.g., grams, liters, molecules).
This systematic approach ensures that chemists can predict and control the outcomes of chemical reactions accurately, whether in a laboratory setting or in large-scale industrial processes.
The Mole and Molecular Weight
In chemistry, understanding the relationship between the amount of substance and its weight is crucial for various calculations, especially when dealing with compounds. This relationship helps chemists quantify the exact proportions of elements within a substance, providing a way to connect atomic-level quantities with macroscopic amounts. Knowing the weight of a specific quantity of atoms or molecules enables scientists to predict and measure chemical reactions more accurately.
Understanding Molecular Weight
Molecular weight refers to the sum of the atomic weights of all the atoms in a molecule. It is an essential concept for determining how much of a substance is needed in a reaction or how much product will be formed. Each element in a compound contributes to the overall molecular weight based on its atomic mass, which can be found on the periodic table. The greater the molecular weight, the heavier the molecule.
- Example: Water (H₂O) has a molecular weight of approximately 18.015 g/mol, as each hydrogen atom contributes 1.008 g/mol and the oxygen atom contributes 16.00 g/mol.
- Example: Carbon dioxide (CO₂) has a molecular weight of about 44.01 g/mol, with carbon contributing 12.01 g/mol and each oxygen atom contributing 16.00 g/mol.
Using Molecular Weight in Calculations
Once the molecular weight of a compound is known, it becomes easier to calculate how many moles of a substance are present in a given mass. This allows scientists to convert between mass and the number of molecules or atoms involved in a reaction. By using molecular weight, chemists can ensure the correct amount of each reactant is used, optimizing chemical processes and reducing waste.
How to Find the Number of Moles

Determining how much of a substance is present in terms of atomic or molecular quantity requires specific calculations. This process is important when working with chemical reactions, as it allows scientists to measure substances in quantities that are manageable in a laboratory setting. By knowing the weight of a substance and its molecular composition, the number of particles or entities can be calculated effectively.
Steps to Calculate Amount of Substance
To find how many units are present in a given mass of a substance, you must use the substance’s molecular weight and the mass you have. The calculation is straightforward once you know these values. Below is the general formula:
| Formula | Explanation |
|---|---|
| Number of Moles = Mass / Molar Mass | Divide the given mass of the substance by its molar mass (molecular weight). This will give the number of moles of the substance. |
Example Calculation
Suppose you have 36 grams of water (H₂O). The molar mass of water is approximately 18.015 g/mol. Using the formula:
| Given Data | Values |
|---|---|
| Mass of Water | 36 g |
| Molar Mass of Water | 18.015 g/mol |
| Number of Moles | 36 g / 18.015 g/mol ≈ 2 moles |
Therefore, you have approximately 2 moles of water in the sample. This method applies to any substance once you have its molar mass and mass value.
The Role of the Mole in Gas Laws
Understanding how gases behave under various conditions is crucial in chemistry and physics. To predict and explain these behaviors, scientists use specific laws that relate pressure, volume, and temperature of gases. One key factor in these equations is the quantity of particles present in a gas, which is directly linked to the concept of a specific unit used to count entities at the molecular level. This unit allows chemists to calculate how gases will react to changes in temperature and pressure, providing a foundation for the ideal gas law and other related principles.
Gas Laws and Their Connection to Particles
Several gas laws describe the relationship between different factors, such as temperature, pressure, and volume. These laws are typically expressed as formulas that involve the number of particles in a given sample. Below are some of the most important relationships:
- Boyle’s Law: States that the pressure of a gas is inversely proportional to its volume, assuming temperature and particle number are constant.
- Charles’ Law: Describes how the volume of a gas expands with increasing temperature when pressure and the number of particles remain constant.
- Avogadro’s Law: Relates the volume of gas to the number of particles, asserting that equal volumes of gases at the same temperature and pressure contain the same number of particles.
- Ideal Gas Law: Combines several gas laws into one equation: PV = nRT, where “n” represents the quantity of gas particles, linking this quantity to pressure, volume, and temperature.
Impact on Gas Calculations
By knowing the quantity of particles, scientists can accurately predict and measure how a gas will behave in different conditions. For example, in the ideal gas law, the number of particles plays a crucial role in determining the behavior of a gas sample. This relationship allows for accurate calculations of the amount of gas in a given volume at specific pressure and temperature values, which is essential in industries like medicine, engineering, and environmental science.
The History Behind the Mole Concept
The concept of quantifying entities at the atomic and molecular level has evolved over centuries. Scientists needed a way to count and describe large quantities of particles that were too small to observe directly. The development of this idea began long before the formalization of the unit we use today. Key discoveries and advancements in atomic theory paved the way for the creation of a system that could bridge the gap between the invisible world of atoms and the macroscopic quantities we encounter in everyday life.
Early Foundations of Atomic Theory
In the early 19th century, the groundwork for modern atomic theory was laid by scientists such as John Dalton. Dalton proposed that elements were composed of indivisible particles called atoms and that each element had its own type of atom. This concept of atoms as fundamental building blocks of matter led to the need for a way to quantify them in a way that could be applied in chemical reactions.
Avogadro’s Contribution
One of the most important milestones in this journey was the work of Amedeo Avogadro, an Italian scientist. In 1811, Avogadro postulated that equal volumes of gases, at the same temperature and pressure, contain the same number of particles. This was a revolutionary idea because it introduced the notion that the number of particles could be directly related to volume and provided a way to calculate the number of particles in a given gas sample. This concept would later lead to the definition of a constant that bears his name, Avogadro’s number.
Modern Development and Recognition
As atomic theory advanced, the need for a standardized way to quantify particles became increasingly apparent. By the late 19th and early 20th centuries, chemists had developed a more precise understanding of atomic masses, and Avogadro’s constant was recognized as a fundamental part of chemical calculations. In the 20th century, the unit became an essential tool in chemistry, allowing scientists to describe large quantities of particles in a consistent manner. Today, it is integral to calculations in stoichiometry, gas laws, and other areas of chemistry.
Key Milestones in Mole Concept History
| Year | Event |
|---|---|
| 1811 | Amedeo Avogadro proposes that equal volumes of gases contain the same number of particles. |
| 1865 | Johann Josef Loschmidt calculates the number of particles in a given volume of gas, leading to the first estimates of Avogadro’s number. |
| 1909 | Millikan’s oil drop experiment allows a more accurate determination of Avogadro’s number, providing a practical value for the constant. |
| 1910s-1920s | The concept of using a specific quantity for atomic and molecular counting becomes formalized in scientific practice. |
Common Mistakes in Mole Calculations
When performing calculations related to atomic and molecular quantities, even experienced chemists can sometimes make errors. These mistakes can arise from misunderstandings of fundamental concepts, misapplications of formulas, or simple computational oversights. Recognizing and addressing these common pitfalls is crucial for ensuring accurate results in chemical calculations.
1. Confusing Mass and Amount of Substance
One of the most frequent mistakes is confusing the mass of a substance with its quantity in terms of particles or entities. It’s important to remember that mass is a measure of how much matter an object contains, while the quantity of substance is usually expressed in terms of particles or entities. When working with formulas, be sure to convert between mass and number of particles using the appropriate constants or molar masses.
2. Incorrect Use of Avogadro’s Number
Another common mistake is incorrectly applying Avogadro’s constant. This number represents the number of particles (atoms, molecules, or ions) in one unit of substance, but it must be used in the right context. Some students might accidentally apply it to a mass rather than a quantity of particles, leading to incorrect calculations. It’s essential to understand when and how to use this constant in order to calculate the correct amount of substance.
3. Not Accounting for Units Properly
Units play a vital role in calculations, and neglecting to track them properly can lead to errors. For instance, when converting between grams, moles, and particles, it’s important to ensure all units are consistent throughout the calculation. Often, students forget to convert or misapply unit conversions, which can throw off the final result. Always check that units match up at each step of the calculation process.
4. Misunderstanding Molecular Formulas and Empirical Formulas
Another area where mistakes commonly occur is in understanding the difference between molecular and empirical formulas. The empirical formula represents the simplest whole-number ratio of elements in a compound, while the molecular formula indicates the actual number of atoms in a molecule. Confusing these two can lead to incorrect calculations of the amount of substance or particles present.
5. Rounding Errors
Finally, rounding errors can significantly impact the accuracy of calculations. While rounding may be necessary at times, it’s important to wait until the final step of a calculation before rounding off the result. Rounding too early in a multi-step process can lead to substantial inaccuracies in the final outcome.