
In this section, we explore key concepts related to the movement of substances across cell membranes, focusing on how environmental conditions and molecular interactions influence these processes. Through interactive simulations, learners can observe the mechanisms that govern how molecules travel in different settings and under varying conditions.
These simulations provide an engaging way to study the dynamics of fluid movement, concentration gradients, and molecular behavior in controlled environments. By experimenting with different parameters, students can gain a deeper understanding of the forces at play and how they impact biological systems.
Key insights are drawn from observing these movements, offering students a practical approach to complex biological phenomena. Whether it’s understanding how substances reach equilibrium or how temperature affects molecular speed, these activities allow for a hands-on learning experience.
Virtual Osmosis and Diffusion Lab Activity Answers
This section focuses on the key observations and outcomes of the simulated experiments related to the movement of molecules across membranes. By exploring different experimental setups, learners can gain insights into how various factors influence the transport of substances in biological systems. Each simulation helps reveal how changes in concentration, temperature, and other conditions affect molecular behavior.
Key Results from the Experiment
Through simulation, the following outcomes are typically observed:
- Changes in the rate of movement based on concentration gradients
- Temperature’s influence on molecular speed and movement
- The impact of different membrane properties on substance passage
- The role of solute size in determining passage rates
Common Observations
During the experiment, several common patterns emerge:
- Substances move more rapidly at higher temperatures, as molecules gain energy.
- The direction of movement tends to follow the gradient, with molecules moving from areas of higher to lower concentration.
- Membrane permeability plays a critical role in controlling which substances can pass through.
- Smaller molecules or ions typically diffuse more easily across barriers than larger molecules.
By analyzing these patterns, students can better understand the mechanisms that control the flow of substances in living organisms. These findings also provide a foundation for exploring more complex processes related to cellular functions.
Understanding Osmosis in Virtual Labs
This section explores how the movement of water molecules across semi-permeable membranes is modeled and analyzed using interactive simulations. By altering key factors like concentration gradients and membrane permeability, learners can observe how changes affect the overall flow of substances and understand the forces behind these movements. The experiments provide a hands-on approach to studying one of the most fundamental processes in biology.
In these simulations, students can manipulate environmental conditions and track the outcomes in real time, making it easier to visualize how molecular transport works. By testing different setups, the dynamics of fluid movement become clearer, revealing how cells manage material exchange under various circumstances.
Key Variables Influencing the Process
The following factors play an important role in determining how molecules move across barriers:
| Factor | Effect |
|---|---|
| Concentration Gradient | A higher gradient accelerates the movement from high to low concentration areas. |
| Membrane Permeability | Determines the ease with which molecules can pass through the barrier. |
| Temperature | Increased temperature speeds up molecular movement. |
| Molecule Size | Smaller molecules tend to pass through more easily than larger ones. |
By manipulating these variables, students gain a deeper understanding of how water and other substances move within living organisms. Such insights help explain critical biological functions, including nutrient absorption and waste removal in cells.
How Diffusion Affects Molecular Movement

This section delves into how the movement of particles within a given space is influenced by concentration differences. As substances naturally move to balance concentrations across membranes or barriers, the factors that drive this process become crucial for understanding molecular behavior. By examining this phenomenon, students can grasp how materials spread throughout various environments, including biological systems.
In experiments that simulate these processes, the rate of movement varies based on several key factors. Altering these variables allows learners to see how changes in environmental conditions can affect the speed and direction of substance movement, offering valuable insights into how cells manage material exchange.
Factors Affecting Molecular Movement

The following table summarizes the main factors that impact the rate and extent of molecular movement:
| Factor | Effect on Movement |
|---|---|
| Concentration Difference | A larger concentration gradient increases the speed of molecular movement as particles move from high to low concentration areas. |
| Temperature | Higher temperatures cause molecules to move faster, enhancing the rate of spreading or mixing. |
| Size of Molecules | Larger molecules move slower compared to smaller ones, affecting how quickly they can reach equilibrium. |
| Medium Density | Denser mediums, such as thick liquids, slow down molecular movement compared to less dense ones like gases. |
By adjusting these factors, learners can better understand how materials naturally balance within cells and other environments. This process plays a vital role in maintaining homeostasis, allowing organisms to efficiently exchange gases, nutrients, and waste products.
Key Variables in Osmosis Experiments
In these experiments, several factors influence how substances move across barriers. By adjusting these key variables, students can better understand the underlying mechanisms that control molecular movement in biological systems. Each variable can significantly affect the results, making it crucial to observe and measure them carefully to draw accurate conclusions.
The main factors that govern the process include concentration gradients, temperature, membrane properties, and the size of the molecules involved. These elements determine how substances travel from one area to another, either by natural movement or through active transport processes. Manipulating these conditions helps simulate different biological environments and study their impact on material exchange within cells.
Important Variables to Consider
The following table highlights the critical variables to monitor during experiments:
| Variable | Effect on Movement |
|---|---|
| Concentration Gradient | The greater the difference in concentration, the faster the movement of molecules across the membrane. |
| Temperature | Higher temperatures increase the speed of molecular motion, enhancing the rate at which substances move. |
| Membrane Permeability | How easily a substance can pass through the membrane depends on its permeability. More porous membranes allow faster movement. |
| Molecule Size | Larger molecules encounter more resistance, moving more slowly than smaller ones. |
Understanding how each of these factors interacts within the experiment allows students to predict the outcome of various setups and draw conclusions about the processes at play. These insights are essential for understanding cellular functions in real-world biological systems.
The Role of Concentration Gradients
Concentration differences across membranes are fundamental in driving the movement of substances in living systems. When molecules are distributed unevenly, they naturally move from areas of higher concentration to regions of lower concentration in an attempt to achieve balance. This process plays a key role in various biological functions, such as nutrient absorption and waste removal in cells.
The gradient between two areas dictates the direction and rate of molecular movement. The greater the difference in concentration, the more rapid the movement of molecules. Understanding how these gradients function helps explain how cells maintain homeostasis and regulate internal conditions despite external changes.
How Concentration Affects Movement
In biological systems, concentration gradients are vital in controlling the flow of substances. Some important factors to consider include:
- Steep Gradients: Larger concentration differences lead to faster molecular movement, as molecules move to reach equilibrium.
- Equilibrium: Once the concentration is balanced on both sides of a membrane, molecular movement slows down, with equal rates of movement in both directions.
- External Factors: Temperature, pressure, and medium density can all influence the effectiveness of a concentration gradient in driving movement.
The Importance of Maintaining Gradients
For cells to function properly, maintaining specific concentration gradients is essential. For example, ion gradients across cell membranes are critical for nerve impulses and muscle contraction. Disrupting these gradients can have serious biological consequences, making them an essential part of cellular homeostasis.
Impact of Temperature on Diffusion
Temperature plays a crucial role in the movement of particles, as it directly affects the kinetic energy of molecules. When the temperature increases, molecules move faster, leading to a higher rate of transport across membranes. Conversely, lower temperatures slow down molecular motion, reducing the speed at which substances spread out. This principle is essential in understanding how various environmental factors can influence the efficiency of material exchange within biological systems.
In experiments, temperature changes can dramatically alter the rate of substance movement, providing valuable insights into how temperature affects natural processes like respiration, nutrient uptake, and waste removal in living organisms. By manipulating temperature, learners can see firsthand how it impacts the rate at which substances move from one region to another.
Effects of Increased Temperature
As temperature rises, the energy of molecules increases, which accelerates their motion. This leads to:
- Faster Movement: Molecules collide more frequently, causing them to move more quickly across barriers.
- Higher Spread Rate: The greater molecular movement enhances the speed at which substances distribute in a given environment.
- Increased Equilibrium Speed: The time taken for substances to reach equilibrium is reduced at higher temperatures.
Effects of Lower Temperature
When the temperature decreases, molecular movement slows down, resulting in:
- Slower Movement: Molecules move more slowly, leading to reduced collision frequency and a lower rate of spreading.
- Prolonged Equilibrium Time: The process of reaching balance takes longer, as the slower movement reduces the overall rate of transfer.
Understanding the impact of temperature on molecular movement is crucial for explaining how environmental conditions affect biological processes. It highlights the importance of maintaining optimal temperature conditions for efficient cell function.
Virtual Lab Simulation Tools Explained
Simulation tools provide an interactive environment where students can model biological processes and experiment with different variables to observe their effects. These digital platforms allow users to manipulate settings, visualize results in real time, and gain insights into the movement of substances across membranes and other biological barriers. They serve as valuable learning aids for those who wish to explore complex scientific principles in a controlled and flexible setting.
By using these tools, learners can adjust various parameters, such as concentration gradients, temperature, and membrane permeability, to see how each one influences the outcome. The flexibility of these simulations makes them ideal for exploring theoretical concepts in a way that traditional hands-on experiments might not allow.
Key Features of Simulation Tools
The following table highlights the essential features that enhance the learning experience when using simulation tools:
| Feature | Description |
|---|---|
| Real-Time Results | Instant feedback on how changes to variables affect the movement of substances within the simulated environment. |
| Variable Manipulation | Allows users to adjust key factors like temperature, concentration, and permeability to see how they influence outcomes. |
| Visualization Tools | Graphs, charts, and diagrams that help users better understand the effects of different conditions on molecular movement. |
| Interactive Controls | User-friendly interface that makes it easy to set up experiments, adjust settings, and track results. |
| Data Logging | Record keeping of experiment data for analysis, comparison, and future reference. |
These simulation tools enable learners to experiment freely without the need for physical materials, offering an engaging and efficient way to study complex biological processes. By understanding how various factors impact molecular movement, students can develop a deeper understanding of cellular functions and biological mechanisms.
Common Errors in Osmosis Lab Activities
In scientific experiments, especially those involving complex processes like molecular movement, errors can often arise that affect the accuracy of results. These mistakes may occur during setup, data collection, or interpretation of findings. Identifying and understanding these common errors is essential for improving experimental techniques and ensuring valid conclusions.
By carefully considering potential sources of error, students can refine their approach and enhance the reliability of their observations. Below are some of the most frequent issues encountered during experiments related to molecular transport across membranes:
Typical Mistakes in Experimental Setup
- Incorrect Concentration of Solutions: Using incorrect concentrations of solute can lead to inaccurate results and misinterpretation of how substances move.
- Poorly Prepared Membranes: If the membrane is damaged or improperly positioned, it can interfere with the intended movement of molecules, leading to skewed results.
- Inconsistent Temperature Control: Failing to maintain a consistent temperature can significantly affect the rate of movement, introducing variability into the results.
- Improper Timing: Failing to accurately measure time intervals can result in inaccurate data about how long it takes for substances to reach equilibrium.
Issues in Data Collection and Analysis
- Inaccurate Measurements: Using the wrong tools or techniques for measuring the amount of movement or concentration can lead to unreliable data.
- Failure to Control All Variables: Not controlling all relevant factors, such as light exposure or humidity, may affect the outcome of the experiment.
- Misinterpretation of Results: Drawing conclusions based on incomplete or incorrectly analyzed data can lead to misunderstandings about the process being studied.
By being aware of these potential mistakes, students can take proactive steps to minimize their occurrence. Careful preparation, accurate measurements, and consistent conditions are crucial for obtaining reliable results and fully understanding the processes at play.
Analyzing Results from Osmosis Experiments
After conducting experiments involving molecular transport, the next critical step is analyzing the gathered data to draw meaningful conclusions. The ability to interpret results accurately is vital in understanding the underlying processes that govern molecular movement across biological membranes. By examining data carefully, students can identify patterns, assess the influence of various factors, and confirm the validity of their hypotheses.
Key aspects to consider when reviewing experimental outcomes include how substances move, the role of environmental factors, and whether the results align with theoretical expectations. Below are some essential steps and tips for analyzing experimental results effectively:
Steps to Analyze Experimental Data
- Review Collected Data: Ensure all data points are recorded accurately, paying close attention to timing, concentration, and environmental conditions.
- Identify Patterns: Look for trends or patterns in the movement of molecules. For example, did the rate of movement increase with temperature, or did it slow down with higher concentration gradients?
- Compare with Hypotheses: Check if the observed results match the predicted outcomes. Any discrepancies should be analyzed and considered for potential errors or new insights.
- Calculate Averages and Trends: Use averages to smooth out variations and identify consistent trends across multiple trials.
Common Factors to Consider
- Concentration Gradients: Analyze how different concentrations of substances on either side of the barrier affected the rate of transport.
- Temperature Impact: Consider how temperature changes influenced the speed of molecular movement and whether results align with expected patterns.
- Membrane Permeability: Evaluate how changes in the permeability of the membrane, whether natural or simulated, impacted the movement of molecules.
- Time Intervals: Determine if the time intervals were adequate to observe full molecular movement, especially when substances reach equilibrium.
By methodically analyzing the data, students can deepen their understanding of the factors that influence molecular movement. This approach also helps in refining future experiments and improving the accuracy of scientific conclusions.
How Virtual Labs Mimic Real-World Processes
Simulated environments have become an essential tool for studying complex biological mechanisms that are difficult or costly to observe in real life. These digital platforms allow users to replicate real-world phenomena, offering an interactive space where variables can be controlled and manipulated. The accuracy of these simulations helps bridge the gap between theory and practice, making it easier for students and researchers to understand biological processes that occur at the molecular level.
By creating digital models of biological environments, these simulations offer a safe and controlled space for users to explore different scenarios and observe outcomes in real time. This not only aids in understanding how molecules move and interact but also provides insight into how external factors, like temperature or concentration, can influence these processes. Although these simulations do not replicate every aspect of reality, they offer a valuable approximation, allowing users to experiment with various conditions and gain deeper insights into biological behavior.
Effects of Membrane Permeability in Diffusion
The ability of a membrane to allow substances to pass through it plays a crucial role in the movement of molecules across biological barriers. Membrane permeability determines how easily different molecules can move between compartments, which directly affects the rate at which substances reach equilibrium. Variations in membrane structure, composition, and environmental factors can all influence the movement of molecules, making the study of permeability essential for understanding transport processes in living systems.
When a membrane is more permeable, substances are able to pass through more easily, leading to faster equilibrium times. Conversely, a less permeable membrane restricts the movement of molecules, slowing down the process. Understanding these dynamics is vital, as they help explain how cells regulate the intake and removal of various substances, from nutrients to waste products. Factors such as temperature, membrane lipid composition, and the size of the molecules involved all contribute to how effectively a membrane facilitates molecular movement.
Calculating Osmosis Rates in Virtual Labs
In experiments involving molecular transport across selective barriers, calculating the rate at which molecules move between two compartments is essential for understanding the underlying process. In these digital simulations, users can measure how fast substances travel, helping to assess how various factors such as concentration, temperature, and membrane properties influence the rate of movement. The ability to perform these calculations accurately allows for a more comprehensive understanding of molecular behavior in controlled environments.
Key Factors Influencing Rate Calculation

- Concentration Difference: The greater the disparity in concentration between two sides of the barrier, the faster the rate at which molecules tend to move from high to low concentration.
- Temperature: Higher temperatures generally increase the energy and speed of molecular movement, resulting in faster transport rates.
- Membrane Properties: The permeability and structure of the membrane significantly affect the rate, as more permeable membranes allow quicker movement of molecules.
Calculating the Movement Rate
- Formula for Rate Calculation: The rate can be expressed as the change in mass or volume over time. For example, the formula Rate = (Final value – Initial value) / Time can be applied to determine how quickly substances move across the barrier.
- Measuring Units: The units for rate typically involve a measure of mass (grams) or volume (liters), divided by time (seconds or minutes).
- Data Interpretation: Once the rate is calculated, interpreting the results helps in drawing conclusions about how external factors, such as temperature or membrane permeability, impact molecular transport.
By calculating the transport rate accurately, users can gain valuable insights into how various factors influence the speed of molecular movement, enabling them to predict outcomes and better understand the process in a scientific context.
Understanding the Importance of Control Variables
In any scientific experiment, it is crucial to account for factors that could influence the results outside of the variables being tested. These factors, known as control variables, must be kept constant throughout the experiment to ensure that the changes observed are solely due to the manipulated variables. Without proper control of these factors, it becomes difficult to draw accurate conclusions about the relationship between the tested variables and the observed outcomes.
Key Control Variables in Transport Studies
- Temperature: Fluctuations in temperature can affect the movement of molecules. Keeping it constant ensures that any observed changes are not due to heat variation.
- pH Level: The pH of the environment can alter the charge and shape of molecules, potentially influencing their movement across barriers. Maintaining a consistent pH is essential for accurate results.
- Concentration of Solvents: Variations in the concentration of solvents can affect the rate of transport. A controlled concentration ensures that any changes observed are not due to solvent differences.
Ensuring Accuracy and Consistency
- Minimizing External Interference: External factors such as air pressure or light conditions can interfere with the results. By keeping these factors constant, experiments become more reliable.
- Replication: Repeating the experiment multiple times under the same controlled conditions helps verify that the results are consistent and not caused by uncontrolled variables.
By carefully managing control variables, researchers can isolate the effect of the manipulated variables, leading to more reliable and reproducible results. This practice is essential for producing scientifically valid data and drawing meaningful conclusions in any transport-based experiment.
How Solute and Solvent Interact in Virtual Labs

In experiments involving molecular transport, understanding the interaction between solutes and solvents is fundamental. The solute, typically a substance like salt or sugar, dissolves in a solvent, such as water, to form a solution. This interaction plays a crucial role in determining how substances move across barriers or membranes. The way solute particles spread out in a solvent is a key factor that influences many biological and chemical processes. In controlled simulations, this process can be closely examined to understand how different conditions affect the movement and distribution of solutes.
Factors Affecting Solute-Solvent Interaction
- Concentration: The higher the concentration of solute, the more particles there are to move and interact with the solvent molecules, affecting the rate of spread.
- Temperature: As temperature increases, the kinetic energy of molecules rises, causing the solute particles to disperse faster through the solvent.
- Polarity: The polarity of both the solute and solvent determines how easily they will mix. Polar solvents typically dissolve polar solutes, while non-polar solvents dissolve non-polar solutes.
Modeling Solute-Solvent Interactions
In a controlled simulation, factors like solute concentration, temperature, and polarity can be adjusted to observe how they influence the movement of solute particles. By using virtual tools, it is possible to simulate these conditions accurately, providing insights into how substances behave in real-world scenarios. These simulations allow students and researchers to manipulate variables that are difficult or impractical to alter in physical experiments.
| Condition | Effect on Solute Movement |
|---|---|
| High Concentration | Faster solute dispersion as more particles are available to move through the solvent. |
| High Temperature | Increased molecular movement leads to faster diffusion of the solute through the solvent. |
| Polar Solvent | Polar solutes dissolve and disperse more effectively due to similar molecular properties. |
By analyzing how solutes interact with solvents in simulations, valuable insights can be gained about molecular movement, which is essential for understanding processes like nutrient transport in cells or the behavior of substances in different chemical environments.
Challenges of Virtual Lab Simulations
While digital tools provide an efficient way to model complex scientific processes, they come with a set of challenges. Simulating biological or chemical systems can never fully replicate the unpredictable variables of real-world experiments. The accuracy of the results depends on the assumptions, algorithms, and data used in the simulation, which may not always align with real-life conditions. Furthermore, participants may encounter difficulties when trying to interpret results that are influenced by controlled variables that don’t have the same level of complexity as in actual environments.
Limitations of Virtual Simulations
- Inaccurate Real-World Replication: Simulations often oversimplify complex systems, omitting environmental factors that may influence outcomes in real experiments.
- Lack of Hands-On Experience: Virtual tools cannot provide the tactile experience of handling real equipment, which is often essential for understanding practical challenges.
- Over-Reliance on Pre-Set Parameters: The pre-programmed settings in simulations may limit the range of variables that can be manipulated, hindering the exploration of broader scientific questions.
Potential for Misinterpretation
- Misleading Results: If the simulation is not correctly calibrated or if the user does not fully understand the input parameters, it can lead to results that do not reflect real-world behavior.
- Difficulty in Understanding Variability: Virtual platforms often cannot simulate the natural variability found in biological and chemical systems, which can mislead learners into expecting consistent outcomes in all conditions.
- Over-Simplified Scenarios: In some cases, simulations present idealized situations that may not account for the full complexity of real-world phenomena, which can distort understanding.
Despite these challenges, digital simulations are still valuable tools in education and research. They allow users to test hypotheses and explore different scenarios quickly. However, it is important to recognize their limitations and complement virtual learning with physical experimentation when possible to achieve a more comprehensive understanding of scientific principles.
Linking Diffusion and Osmosis to Biology
The movement of molecules across membranes plays a crucial role in the functioning of living organisms. These processes are fundamental to many biological functions, such as nutrient uptake, waste removal, and cellular communication. By understanding how substances naturally spread from areas of high concentration to areas of low concentration, we can gain insight into vital physiological mechanisms that sustain life. These principles are central to cellular functions in all living organisms, from single-celled organisms to complex multicellular beings.
Role in Cellular Processes
- Nutrient Absorption: Cells rely on the natural flow of molecules across their membranes to absorb essential nutrients, like glucose and amino acids, from their surroundings.
- Waste Removal: Cells expel waste products, such as carbon dioxide and urea, through the same processes, helping to maintain homeostasis.
- Gas Exchange: In organisms like plants and animals, the movement of oxygen and carbon dioxide across cell membranes is vital for respiration and photosynthesis.
Impact on Homeostasis
- Fluid Balance: The regulation of water movement is key to maintaining the proper balance of fluids within cells, tissues, and organs.
- Temperature Regulation: Cellular processes such as enzyme activity and metabolism depend on the proper regulation of heat, which is influenced by the movement of molecules.
- Acid-Base Balance: The movement of ions across membranes helps maintain the pH levels necessary for cellular function.
By studying these movements in a controlled environment, scientists can better understand how cells adapt to changing conditions and how they interact with their surroundings. The insights gained from this understanding have profound implications for fields like medicine, agriculture, and environmental science, where the principles of molecule movement are applied to improve health, productivity, and sustainability.
Learning Outcomes from Osmosis Virtual Lab
Through interactive simulations that replicate real-world processes, students can gain a deeper understanding of how substances move across membranes. These simulations offer a unique opportunity to visualize concepts that are otherwise difficult to observe in traditional classroom settings. By engaging with such activities, learners can explore the principles of selective permeability, concentration gradients, and the impact of environmental factors on molecular movement.
Key Concepts Gained
- Understanding Movement Across Membranes: Learners can observe how molecules naturally move from areas of higher concentration to areas of lower concentration, facilitating a better understanding of cellular processes.
- Exploring the Role of Solutes and Solvents: These tools help to visualize how solutes interact with solvents, enabling learners to understand the fundamental concepts of concentration, equilibrium, and the driving forces behind molecular movement.
- Recognizing the Importance of Variables: Students can experiment with different variables, such as temperature, concentration, and membrane permeability, to observe their effects on the rate and direction of molecular movement.
Practical Applications
- Real-World Relevance: Knowledge gained from these simulations can be applied to various biological systems, such as nutrient uptake in cells, kidney function, and plant water regulation.
- Enhanced Critical Thinking: Students learn to hypothesize, test, and analyze results, strengthening their problem-solving skills and scientific reasoning.
- Improved Experimental Design Skills: These experiences foster the ability to plan experiments, identify key variables, and draw conclusions based on controlled observations.
Ultimately, these learning outcomes provide students with a comprehensive understanding of vital biological processes, enriching their educational journey and preparing them for more advanced studies in the field of biology and other sciences.
Real-World Applications of Osmosis and Diffusion
Understanding the movement of molecules across membranes plays a crucial role in various biological and industrial processes. These mechanisms are not only fundamental to life but also have numerous practical applications that impact everything from medicine to agriculture. By studying how substances naturally move from areas of higher to lower concentration, we can better comprehend how organisms maintain balance and optimize efficiency in complex systems.
Biological Significance
- Cellular Function: In living organisms, the process of substances crossing cell membranes is essential for nutrient uptake, waste removal, and maintaining homeostasis. For example, water absorption in plants and the regulation of salt in animals rely heavily on these natural processes.
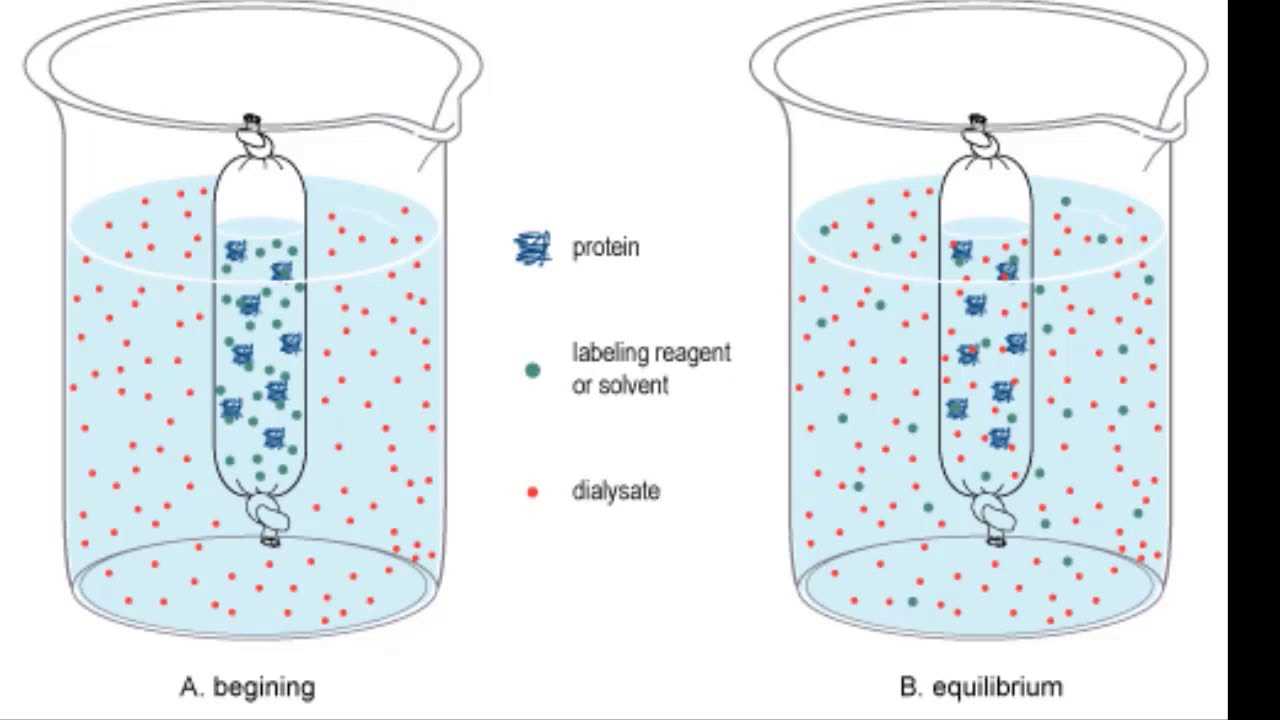
- Medical Applications: Techniques like dialysis use these principles to help filter blood in patients with kidney failure. The selective permeability of membranes in these medical devices ensures that waste products are removed while essential nutrients remain in the blood.
- Drug Delivery: Controlled release of medications is achieved through the understanding of molecular movement. Drugs are designed to diffuse through membranes at specific rates to ensure optimal therapeutic effects over time.
Industrial and Environmental Applications
- Water Purification: Reverse osmosis is commonly used in water filtration systems to remove contaminants. By applying pressure to water, this method forces molecules through a semi-permeable membrane, leaving impurities behind.
- Food Preservation: The movement of water in food products, such as fruits and vegetables, affects shelf life. Techniques like dehydration and salting rely on these natural processes to preserve food by reducing the water content and preventing microbial growth.
- Wastewater Treatment: In industrial settings, wastewater treatment facilities utilize diffusion-based processes to separate harmful substances from water, ensuring that the final effluent is safe for discharge or reuse.
The principles of molecular movement are deeply embedded in both natural systems and human-made technologies. By exploring how these processes work in the real world, we can better understand their critical roles in maintaining life and advancing technological innovations across a wide range of fields.