The movement of molecules within biological systems plays a crucial role in maintaining cellular functions. This process is essential for the survival of cells, allowing them to exchange vital substances with their environment. Whether it involves water or other molecules, understanding how substances move across cell membranes is fundamental to grasping the behavior of living organisms at the microscopic level.
Passive transport processes enable molecules to move naturally without the need for energy input, following concentration gradients. These movements can be driven by various factors, including temperature, concentration differences, and the permeability of the membrane. By exploring different examples, one can better understand the mechanisms behind these vital processes.
In this section, we will explore several methods used to study how substances move in biological systems. With detailed explanations and examples, we aim to shed light on the principles that govern cellular processes. Understanding these mechanisms is key to further learning in biology, helping to clarify the role of cells in maintaining life.
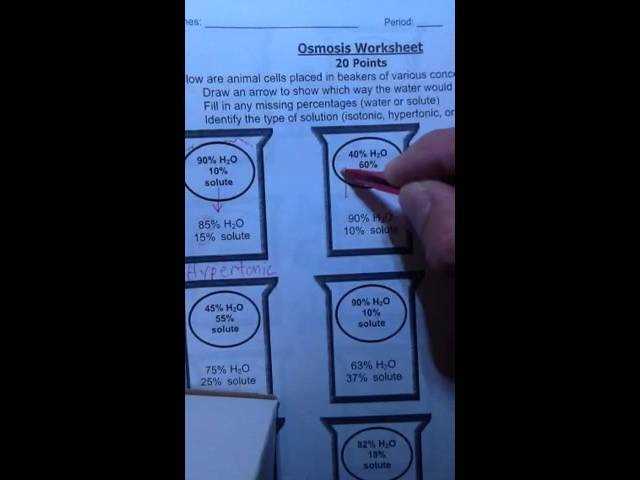
Osmosis and Diffusion Practice Worksheet Answers
Understanding how substances move in and out of cells is essential for grasping key biological processes. This section provides detailed explanations for common scenarios where molecules move across cell membranes. By analyzing specific examples, learners can develop a deeper understanding of the mechanisms behind this natural movement.
Cellular transport is governed by concentration gradients, temperature, and the permeability of cell membranes. The movement of water and solutes plays a vital role in maintaining cellular balance. Through various problems, one can examine how these factors influence the flow of materials in different environments.
For each scenario, learners will explore how factors like concentration differences impact the movement of particles. Understanding these principles will help in answering questions related to cell behavior, ensuring a strong foundation for further studies in biology. The explanations provided aim to clarify common misconceptions and guide learners through complex concepts.
Understanding Osmosis and Diffusion
In biological systems, the movement of molecules is vital for maintaining life. These processes are fundamental to the function of cells, enabling them to obtain necessary substances while removing waste. The natural movement of materials between different concentrations is crucial for various cellular functions, including nutrient absorption and waste removal.
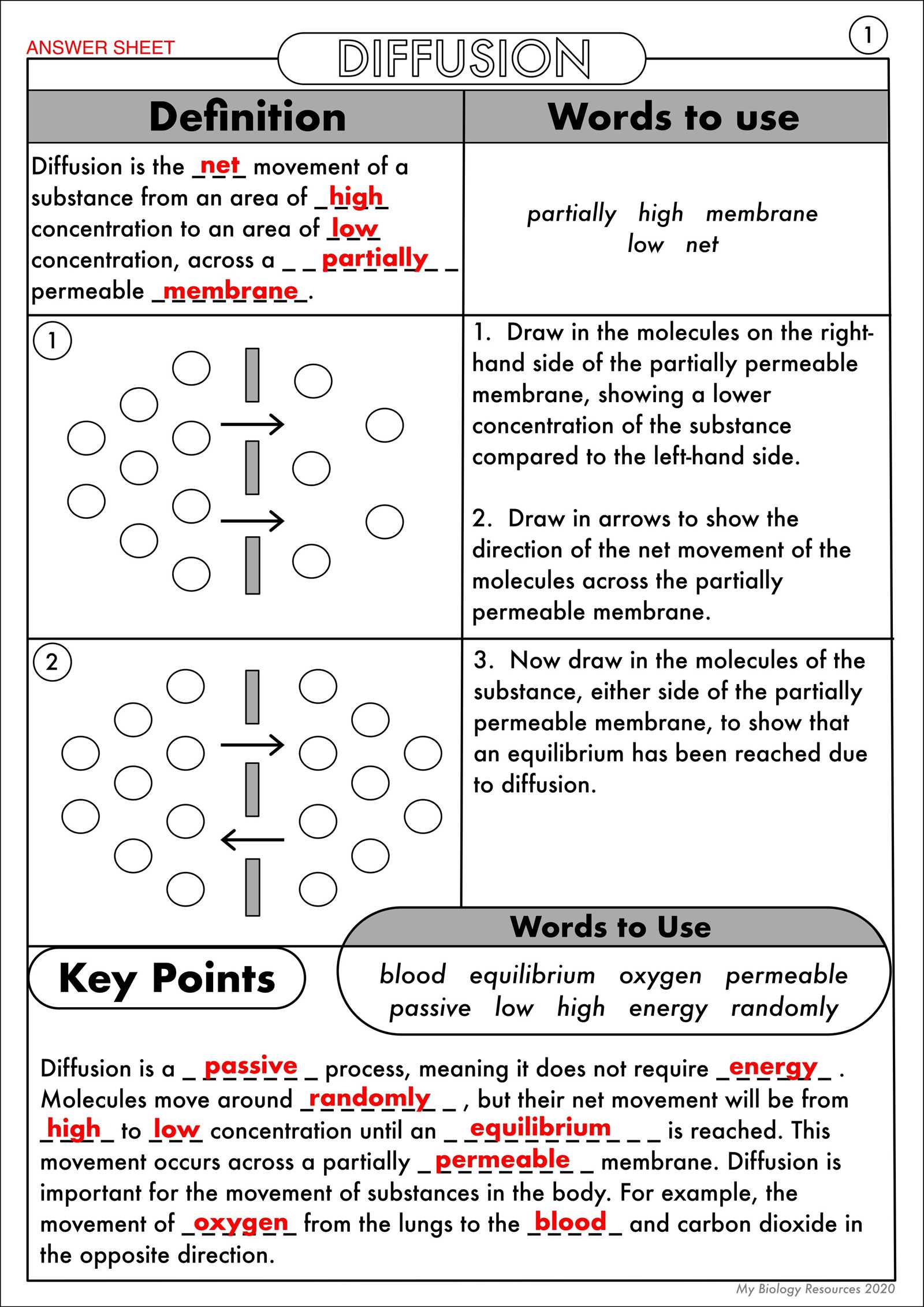
Key Concepts of Molecular Movement
These mechanisms are driven by the concentration differences between two environments, often leading to a balance. Here are some important aspects of molecular movement:
- Concentration Gradient: The difference in concentration of particles between two areas.
- Membrane Permeability: The ability of a membrane to allow certain molecules to pass through.
- Equilibrium: The point at which the concentration of particles is balanced on both sides of the membrane.
Types of Molecular Transport
Different methods exist for the movement of substances across cellular membranes, each with unique characteristics:
- Passive Transport: Movement of substances without energy input, following concentration gradients.
- Active Transport: Movement that requires energy to move substances against their gradient.
- Facilitated Transport: Movement assisted by proteins embedded in the membrane.
Understanding these transport mechanisms is crucial for answering complex biological questions and grasping the fundamental principles of cell physiology. Each process contributes to cellular homeostasis, helping organisms maintain internal balance and respond to changes in their environment.
Key Concepts in Osmosis Explained
In biological systems, the movement of water across cell membranes is essential for maintaining proper cell function. This process ensures that cells receive necessary nutrients and expel waste. Understanding the mechanisms that drive this movement is critical for comprehending cellular processes and the regulation of internal environments.
The Role of Concentration Gradients
The movement of water molecules is influenced by differences in solute concentrations across the membrane. These gradients determine the direction and rate of water flow, ensuring that cells maintain their shape and function. The key concept here is:
- Water Movement: Water naturally moves from areas of lower solute concentration to higher solute concentration until equilibrium is reached.
Impact of Membrane Permeability
Not all substances can freely cross cell membranes. The permeability of the membrane plays a critical role in regulating the movement of water and solutes. This concept is central to maintaining homeostasis within the cell. Some important factors include:
- Selective Permeability: The membrane selectively allows certain molecules to pass while blocking others.
- Hydration of Solutes: The ability of certain solutes to attract water affects the rate at which water moves across the membrane.
Understanding these fundamental principles is crucial for solving problems related to cellular function and ensuring accurate interpretation of how cells maintain their internal environment.
How Diffusion Affects Cells
The movement of particles within a cell or across its membrane is essential for various cellular activities. This process allows cells to acquire nutrients, release waste, and maintain internal balance. Understanding how molecules move naturally helps explain how cells function and adapt to their environment.
Impact on Cellular Function
The natural flow of substances into and out of cells plays a significant role in maintaining homeostasis. It ensures that cells are supplied with the necessary molecules for energy production, metabolism, and other vital processes. Key points include:
- Energy Supply: Cells absorb essential molecules like oxygen and glucose to produce energy.
- Waste Removal: Metabolic waste products are expelled from the cell to prevent buildup.
Role of Concentration Differences
When there is an imbalance in the concentration of substances inside and outside the cell, particles naturally move to equalize the distribution. This movement affects how substances interact with the cell, determining their availability for cellular processes. Important aspects are:
- Concentration Gradient: Substances move from high to low concentration, facilitating the exchange of materials.
- Cellular Efficiency: The rate at which particles move influences how effectively cells perform their functions.
This process is crucial for maintaining proper cell function and adapting to changing environments. Understanding how particles move across membranes helps explain various physiological phenomena in living organisms.
Examples of Osmosis in Nature
The movement of water across cell membranes is a fundamental process in nature, observed in a wide variety of organisms. This natural phenomenon plays a critical role in maintaining the balance of fluids and nutrients within cells, allowing organisms to survive and thrive. Several examples of how this process functions in nature can help illustrate its importance.
- Plant Root Absorption: Water moves from the soil into plant roots through the cell membranes, where it is absorbed into the plant’s vascular system. This ensures that the plant remains hydrated and can transport nutrients throughout its structure.
- Red Blood Cells in Animals: In mammals, red blood cells maintain their shape by balancing the movement of water in and out of the cell. If the surrounding environment is too concentrated or diluted, it can cause the cells to shrink or swell.
- Fish in Saltwater and Freshwater: Fish living in saltwater must regulate water loss due to the higher concentration of salt in their environment. Conversely, freshwater fish work to prevent water from entering their bodies, maintaining a balance of salts and water inside their cells.
- Kidney Function in Humans: The kidneys filter blood, using cellular processes to regulate the balance of water and electrolytes in the body. This allows organisms to maintain proper hydration levels despite changes in their environment.
These examples highlight how crucial the movement of water and solutes is for the survival and function of living organisms. Understanding these processes in nature helps us appreciate the complexity of life at the cellular level.
Types of Diffusion in Biology
In biological systems, molecules move across cell membranes in various ways, each with distinct characteristics and mechanisms. These processes are crucial for the proper functioning of cells, allowing them to exchange materials with their environment. Different types of molecular movement play essential roles in cellular activities and homeostasis.
Passive Transport
This type of movement does not require energy and relies on concentration gradients to move substances from areas of higher concentration to lower concentration. It includes the following mechanisms:
- Simple Movement: Small or nonpolar molecules, like oxygen or carbon dioxide, pass directly through the membrane without assistance from proteins.
- Facilitated Movement: Larger or polar molecules, such as glucose, move through protein channels or carriers embedded in the cell membrane.
Active Transport
Unlike passive transport, this method requires energy (usually in the form of ATP) to move molecules against their concentration gradient, from low to high concentration. Key examples include:
- Ion Pumps: These proteins use energy to move ions, like sodium or potassium, across membranes to maintain ion balance inside cells.
- Endocytosis and Exocytosis: Large molecules are transported into or out of the cell through vesicles, processes that require cellular energy.
These types of movement are essential for maintaining cellular function, allowing cells to absorb necessary nutrients, expel waste, and respond to environmental changes. Understanding the different mechanisms provides insight into how cells interact with their surroundings and maintain internal balance.
The Role of Cell Membranes
The cell membrane serves as a vital barrier that controls the movement of substances into and out of the cell. Its selective permeability ensures that essential molecules, such as nutrients and oxygen, are allowed to enter, while waste products and excess materials are removed. Additionally, the membrane helps maintain the cell’s shape and provides a platform for cellular communication and recognition.
One of the key functions of the cell membrane is to regulate the internal environment of the cell, maintaining a stable balance of ions, nutrients, and water. This ability to selectively allow certain molecules to pass while blocking others is essential for processes such as nutrient absorption, waste removal, and cellular signaling.
| Function | Role in Cell Function |
|---|---|
| Selective Permeability | Controls the entry and exit of substances, ensuring proper internal balance. |
| Communication | Facilitates the interaction between cells through receptor proteins, enabling responses to external signals. |
| Protection | Acts as a protective barrier, preventing harmful substances from entering the cell. |
| Cell Recognition | Helps the cell identify and respond to other cells, important for immune function and tissue formation. |
The cell membrane is not just a static barrier, but a dynamic structure involved in numerous critical functions that allow the cell to interact with its environment and maintain homeostasis. Without this crucial structure, cells would be unable to perform the essential activities required for life.
Factors Influencing Osmosis Rates
The rate at which water moves across cell membranes depends on several factors that influence how efficiently this process occurs. These variables determine how quickly water molecules pass through the membrane, impacting the internal balance of cells. Understanding these factors is essential for comprehending how cells regulate water and solute levels in different environments.
Concentration Gradient
The difference in solute concentration between the inside of the cell and the surrounding environment plays a significant role in determining the speed of water movement. A greater concentration difference results in faster water movement toward the area with higher solute concentration. Key aspects include:
- Steeper Gradient: A more significant difference in solute concentration increases the rate of movement.
- Equilibrium: The process slows as the concentrations balance out, reducing the gradient.
Temperature

The temperature of the environment affects the kinetic energy of water molecules. As temperature increases, molecules move more rapidly, leading to a higher rate of movement. However, extreme temperatures can damage the integrity of the membrane, slowing the process. Key points include:
- Higher Temperatures: Increase molecular movement, resulting in a faster rate of transfer.
- Low Temperatures: Reduce molecular speed, causing a slower process.
These factors, along with others like membrane permeability and the surface area of the membrane, all contribute to how quickly water can move through biological systems, ensuring cells maintain proper hydration and nutrient balance.
Diffusion and Its Impact on Cells
The movement of molecules within and outside of cells is a vital process for maintaining cellular function. This natural tendency of molecules to move from areas of higher concentration to lower concentration ensures that cells can exchange necessary substances, such as nutrients, gases, and waste products. Understanding how this process affects cells is essential for recognizing its role in maintaining homeostasis.
Effects on Nutrient Absorption
The movement of essential nutrients like oxygen and glucose into cells is driven by the concentration gradient. This process allows cells to take in what they need for energy production and other cellular activities. A disturbance in this process can impact cellular health and function.
Waste Removal
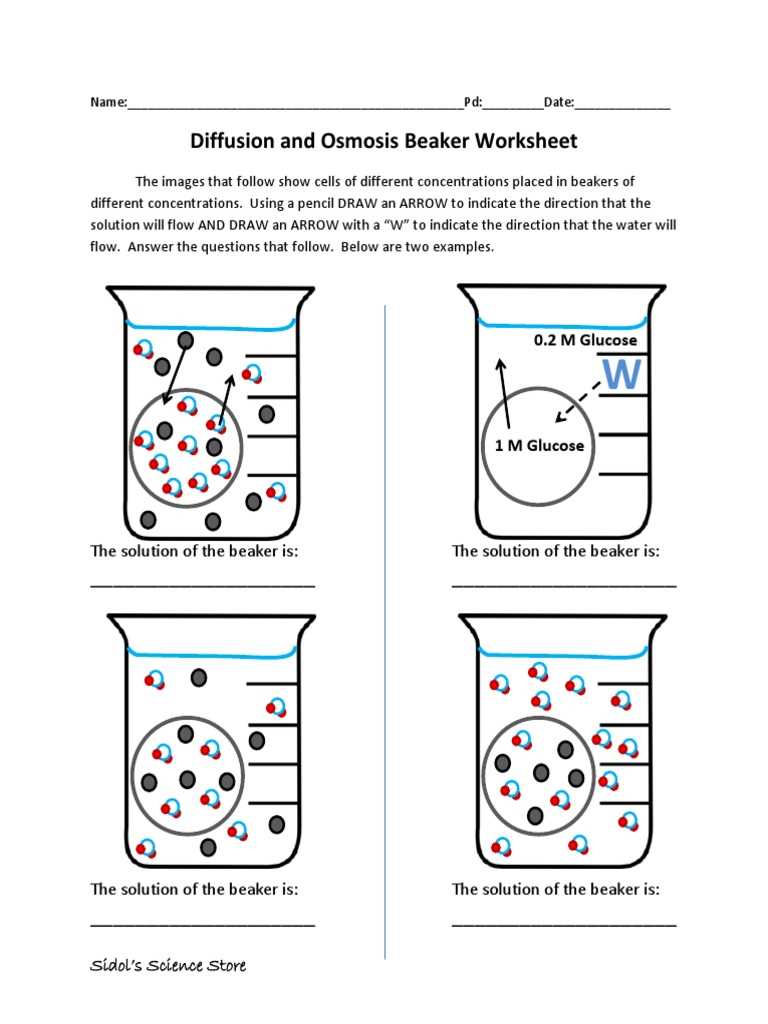
Similarly, waste products such as carbon dioxide are removed from cells via the same mechanism. Efficient removal of waste is crucial for preventing toxic buildup, which can damage cells and disrupt their normal activities.
| Impact | Effect on Cells |
|---|---|
| Increased Nutrient Intake | Ensures cells have the resources needed for energy and growth. |
| Efficient Waste Removal | Prevents toxic accumulation and maintains cellular health. |
| Cellular Communication | Facilitates signaling between cells to coordinate responses to changes in the environment. |
The balance of movement into and out of cells plays a critical role in their overall health and function. Any disruption to this natural process can lead to complications, affecting everything from nutrient absorption to waste management.
Solute Concentration and Osmosis
The concentration of solutes in a solution is a crucial factor influencing the movement of water across a membrane. A higher concentration of solutes in one area creates a difference that drives the movement of water toward that region. This process ensures that cells maintain proper hydration levels and balance necessary substances. Understanding how solute concentration affects the movement of water is vital for comprehending cellular processes.
Impact of High Solute Concentration
When there is a higher concentration of solutes outside the cell than inside, water tends to move towards the area of higher solute concentration. This helps the cell take in the necessary substances for various functions. However, an excess of solutes outside the cell can lead to water loss from the cell, potentially causing it to shrink and impairing its function.
Effect of Low Solute Concentration
In environments where solute concentration is lower outside the cell than inside, water moves into the cell to balance the concentration. If too much water enters, it can cause the cell to swell, and in extreme cases, even burst. Maintaining a balanced environment is crucial for the cell’s survival.
| Solute Concentration | Effect on Water Movement |
|---|---|
| High Outside | Water moves into the area with a higher concentration of solutes. |
| Low Outside | Water moves into the cell to balance the concentration, possibly causing swelling. |
| Equal Concentration | Water movement is balanced, and no significant changes occur in cell volume. |
The concentration of solutes both inside and outside the cell plays a significant role in maintaining the cell’s internal environment. This balance is essential for proper cellular function and overall organism health.
Active vs Passive Transport Explained

Cells rely on different mechanisms to move substances across their membranes. These processes can either require energy or occur naturally without it, depending on the specific needs of the cell. Understanding the distinctions between energy-dependent and energy-independent transport methods is essential for grasping how substances move in and out of cells, ensuring the proper functioning of cellular activities.
Energy-Dependent Transport
In some cases, cells must actively move molecules against a concentration gradient, which requires energy. This process helps maintain the right balance of nutrients, ions, and other substances within the cell. Active transport uses proteins in the membrane and energy derived from ATP to move molecules in the desired direction, even if it means going against the natural flow.
Energy-Independent Transport
On the other hand, some substances can pass through the membrane naturally, without the need for energy. This process occurs when molecules move from areas of higher concentration to lower concentration, following the concentration gradient. It is a passive process that allows cells to regulate their internal environment more efficiently without expending energy.
| Transport Type | Energy Requirement | Movement Direction |
|---|---|---|
| Active Transport | Requires energy (ATP) | Against concentration gradient |
| Passive Transport | No energy required | With concentration gradient |
| Both | Specific proteins are involved | Ensure proper substance balance |
Both types of transport are crucial for cellular health and homeostasis. While active transport helps cells maintain essential gradients, passive transport allows for efficient exchange of substances without the need for energy expenditure.
Real-Life Applications of Osmosis
The movement of water across selective barriers plays a vital role in various everyday processes. From agriculture to medicine, the principles of water balance and concentration differences are applied in numerous real-world situations. Understanding how water moves in different environments allows us to optimize many practical applications that impact daily life.
One of the most common uses of this natural phenomenon is in the preservation of food. For instance, when fruits and vegetables are placed in a solution with a high concentration of sugar or salt, the water inside the cells moves out, helping to preserve the food and prevent spoilage. This principle is used in methods like pickling and curing to keep food fresh for longer periods.
Another example is in the medical field, where understanding this process helps in treating dehydration. Medical professionals often use intravenous (IV) fluids to rehydrate patients. By adjusting the solute concentration of these fluids, healthcare providers can ensure that water moves into the cells in the right amounts to restore proper hydration levels without causing harm.
In the field of agriculture, osmosis plays a crucial role in irrigation techniques. Farmers use osmotic pressure to manage the flow of water into plants, ensuring that crops receive the necessary hydration while minimizing water waste. Techniques like reverse osmosis are also used to purify water for agricultural use, further improving crop yields and conserving resources.
Finally, reverse osmosis is used in water purification systems. This process involves filtering water through a membrane that allows only water molecules to pass through, leaving behind contaminants. It is widely used to provide clean drinking water in homes, industries, and municipalities around the world.
Teaching Osmosis Through Worksheets
One of the most effective ways to help students understand the movement of water across membranes is through interactive exercises. By engaging students with structured activities, teachers can simplify complex biological concepts, making them more accessible and easier to grasp. Using hands-on learning tools allows students to visualize and apply what they learn in practical ways, reinforcing the theory behind the natural processes that occur in cells.
Benefits of Using Educational Exercises
These activities are designed to guide students through key principles while testing their comprehension. Some benefits of using such methods include:
- Visual Learning: Students can observe and document how substances move, solidifying their understanding.
- Critical Thinking: Exercises challenge students to analyze scenarios and predict outcomes based on concentration gradients.
- Engagement: Interactive formats maintain students’ interest and foster a more dynamic classroom environment.
- Application: Students can apply theoretical knowledge to real-world scenarios, connecting science to daily life.
Designing Effective Activities
When designing activities to teach this concept, it is important to consider how to break down the steps for clear understanding. Some ideas include:
- Lab Experiments: Allow students to observe water movement in different environments, like plant cells in saline solutions or in fresh water.
- Case Studies: Provide examples where students can apply their knowledge of concentration gradients and predict the behavior of cells.
- Problem-Solving Scenarios: Present situations where students must decide the optimal method to manipulate water flow in a biological context.
Through these types of activities, students develop a deeper understanding of the processes involved, ensuring they are better prepared to engage with more advanced biological concepts.
Solving Osmosis Practice Problems
Understanding the movement of water across biological membranes requires hands-on problem-solving to reinforce theoretical knowledge. By working through practical scenarios, students can deepen their comprehension and develop the skills needed to predict how different factors influence the process. Solving problems not only tests their grasp of the concept but also strengthens their analytical abilities when applying the principles in various contexts.
Approaching Practical Scenarios
When faced with problems related to the movement of molecules through semipermeable membranes, it is important to break down the steps systematically. Consider these guidelines to help students solve such challenges:
- Identify the Concentration Gradients: Start by determining the relative concentrations of substances on either side of the membrane.
- Consider Environmental Factors: Factors like temperature or pressure can influence the rate at which molecules move.
- Predict the Movement: Based on the concentrations, predict the direction of flow from areas of high to low concentration.
- Measure the Outcome: In experiments, record how much change occurs in the volume or mass of the substances involved.
Examples of Problem Types

Here are some types of problems students can solve to strengthen their understanding:
- Concentration Comparison: Compare the concentration of solutes inside a cell with that outside, predicting the effect on cell volume.
- Water Movement Scenarios: Given different solutions, calculate how water will move across membranes and affect the cell’s size or shape.
- Rate of Transport: Analyze factors such as time and temperature to determine how quickly the movement occurs.
By working through such problems, students learn to apply the principles of cellular transport, reinforcing their understanding through real-life examples and critical thinking.
How to Analyze Diffusion Results

After conducting experiments involving the movement of molecules across cell membranes, it’s essential to carefully analyze the results to gain meaningful insights. The goal is to determine how environmental conditions and different factors affect the rate and direction of molecule movement. By following a structured approach, students and researchers can interpret data and understand the underlying principles behind molecular transport.
Key Considerations for Data Analysis
To effectively analyze results from molecular movement experiments, the following factors should be considered:
- Concentration Gradients: Compare the concentration of molecules on both sides of the barrier. A higher difference typically leads to faster movement.
- Time Duration: Observe how long the process took to reach equilibrium. A longer time might suggest slower movement or barriers in the system.
- Temperature: Note the temperature during the experiment, as warmer conditions often accelerate molecular movement.
- Membrane Permeability: Consider the characteristics of the membrane used in the experiment, as its permeability influences how easily molecules can pass through.
- Volume or Mass Change: Measure any changes in the volume or mass of the substances involved, which can indicate the extent of movement.
Steps for Interpreting the Results
Here are steps to follow when analyzing results:
- Record Initial Data: Start by documenting initial concentrations, volumes, or masses before starting the experiment.
- Track Changes Over Time: Monitor the system at various time points to identify how the concentration or size changes during the experiment.
- Compare Control and Experimental Groups: If possible, use a control group to compare results and identify any significant deviations caused by the experimental conditions.
- Graphical Representation: Create graphs that show changes over time. This will allow for a clearer visual interpretation of how variables affect molecular movement.
- Draw Conclusions: Based on your analysis, determine what factors most strongly influenced the movement of molecules and how they relate to the experiment’s hypotheses.
By carefully analyzing the results, it’s possible to gain a deeper understanding of the factors affecting molecular movement, which can inform further experimentation or theoretical models.
Common Mistakes in Osmosis Problems

When studying the movement of molecules across cell membranes, students often encounter difficulties that can lead to misunderstandings or incorrect conclusions. These challenges typically arise from misinterpreting the process or overlooking important factors that influence the movement. Recognizing these common mistakes can help improve understanding and accuracy in solving problems related to molecular transport.
Overlooking Concentration Differences
One of the most frequent mistakes is neglecting the concentration gradient. The rate of molecular movement is directly influenced by the difference in concentration on either side of a membrane. If the concentration difference is not properly considered, it can lead to incorrect predictions about how substances will move. Always ensure that the concentration of molecules is clearly defined in both areas before proceeding with calculations.
Misunderstanding Membrane Permeability
Another common error involves forgetting to account for the selective permeability of membranes. Not all molecules can pass freely through cell barriers. This characteristic is essential when determining which substances will move and how easily. For instance, larger molecules or charged particles may not cross the membrane as easily as smaller, non-polar ones. Understanding the properties of the membrane is crucial for accurate analysis.
Ignoring Environmental Factors

Temperature and other environmental conditions can significantly affect the rate of molecular movement. Many students fail to consider how changes in temperature might speed up or slow down the process. In general, higher temperatures tend to increase the movement of molecules, while lower temperatures can reduce it. Always note the conditions under which an experiment is conducted to interpret the results accurately.
Incorrect Use of Terms
In some cases, students might confuse related concepts, such as passive vs. active transport or the terms describing the movement of water. It’s important to use the correct terminology to avoid confusion. For example, not distinguishing between the various types of molecular movement (e.g., simple vs. facilitated transport) can lead to misinterpretations of results and faulty conclusions.
Inadequate Time Considerations
Finally, time is an essential factor that should not be overlooked. Many students mistakenly assume that the process of molecular movement will happen instantaneously. However, this process takes time and can vary depending on the conditions. Failing to observe the passage of time during an experiment can lead to premature conclusions, such as assuming equilibrium has been reached too early.
By being mindful of these common errors, students can approach problems more systematically and achieve a better understanding of molecular movement. Ensuring that all relevant factors are considered will lead to more accurate results and a deeper insight into how substances travel across cellular boundaries.