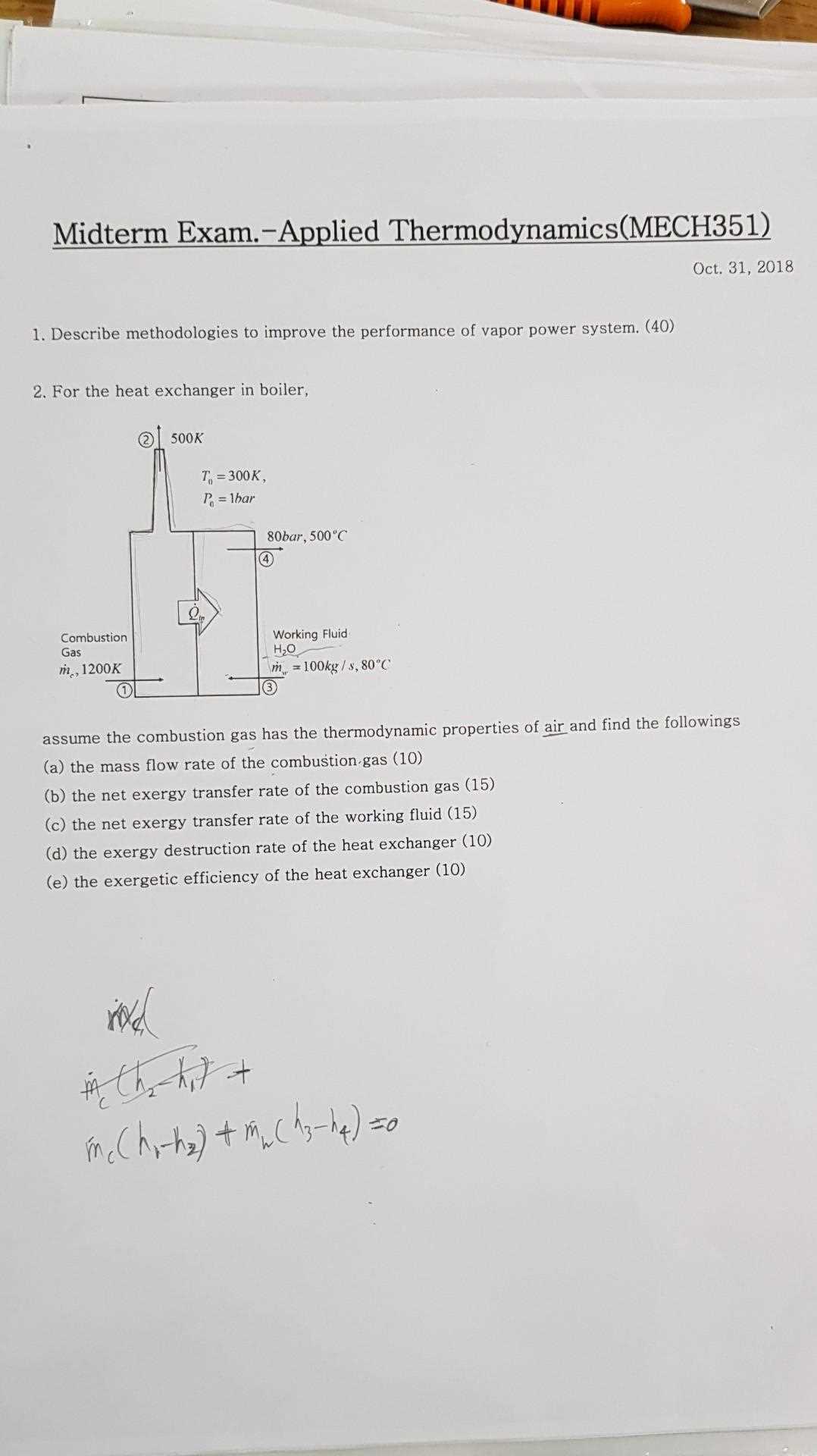
When preparing for tests related to energy transformation and heat management, a clear grasp of core principles is essential. This section will guide you through some of the most significant topics in this field, helping you tackle a variety of problems with confidence.
Key areas like the fundamental laws, efficiency calculations, and the role of entropy are crucial for building a strong foundation. By breaking down complex concepts into manageable parts, you will be better equipped to apply your knowledge in practical scenarios.
In the following sections, we will explore various methods and strategies to approach common challenges, offering insights that can enhance your understanding and performance in related tasks. Whether you’re reviewing essential formulas or tackling intricate calculations, this guide will serve as a valuable resource.
Thermodynamics Exam Questions and Answers
Preparing for assessments related to heat, energy, and work requires a structured approach to understanding key principles. In this section, we’ll cover typical challenges that arise in these types of evaluations and offer strategies to address them effectively. The goal is to enhance your problem-solving skills and boost your confidence in applying theoretical knowledge to practical scenarios.
Key Topics to Focus On
- Energy conversion processes
- Basic thermodynamic laws
- Heat transfer methods
- Entropy and its implications
- Efficiency calculations in systems
Common Problem-Solving Techniques
- Identify known variables and desired outcomes
- Apply relevant equations for energy transformation
- Break complex calculations into smaller, manageable parts
- Verify units and check for consistency
- Use approximations when necessary to simplify the process
By focusing on these fundamental areas and practicing various problem types, you’ll be better prepared to tackle any related topics with precision and clarity. Consistent review of core concepts and refining calculation techniques are key steps in achieving mastery in this field.
Basic Principles of Thermodynamics
Understanding the fundamental concepts related to energy, heat, and work is essential for solving problems in this field. These core principles govern how energy is transferred, transformed, and conserved in different systems. Mastering these ideas will help build a solid foundation for tackling more advanced topics and solving practical challenges.
The First Law of Energy Conservation
This law states that energy cannot be created or destroyed, only converted from one form to another. In any system, the total energy remains constant, though it may change forms, such as from heat to mechanical work or internal energy to external work. Understanding this law is vital for analyzing processes that involve energy conversion.
The Second Law of Entropy
The second law focuses on the concept of entropy, which measures the disorder or randomness of a system. It asserts that the total entropy of an isolated system always increases over time. This principle explains the direction of natural processes, such as heat flowing from a hot object to a cold one, and limits the efficiency of energy conversion systems.
By applying these fundamental laws, you can analyze energy systems and understand the limits and possibilities of various processes. Developing a deep understanding of these concepts is crucial for solving complex problems in related topics.
Common Thermodynamics Topics for Exams
In any assessment of energy, heat, and work, certain topics tend to appear more frequently. These subjects often form the foundation of many problems and are crucial for demonstrating a strong understanding of how energy behaves in different systems. Mastering these areas will ensure that you are well-prepared to tackle various challenges.
Energy Conversion and Efficiency
Understanding how energy is transformed from one form to another, such as from heat to mechanical work, is fundamental. Additionally, calculating the efficiency of these conversions is a key topic, as it determines how much useful energy is obtained from a system in comparison to the total energy input.
Thermodynamic Cycles

Many real-world applications involve cyclic processes, such as heat engines and refrigeration systems. These cycles are critical for understanding how energy is used and regenerated within a system. Familiarity with various types of cycles, such as the Carnot and Rankine cycles, is essential for solving related problems.
Focusing on these common topics will help you develop the necessary skills and knowledge to approach complex scenarios effectively. By practicing calculations and applying theoretical principles, you’ll gain a better understanding of energy systems and their limitations.
Key Concepts in Heat Transfer
Understanding how heat moves within different systems is essential for solving many problems related to energy. Heat can transfer in several ways, each of which plays a significant role in various practical applications. A solid grasp of these concepts will allow you to analyze how energy flows and how it can be controlled or utilized in systems.
There are three primary modes of heat transfer: conduction, convection, and radiation. Each method involves different mechanisms and is applicable to different situations. For example, conduction occurs when heat travels through a solid material, while convection involves the movement of fluids. Radiation, on the other hand, transfers heat through electromagnetic waves, like the warmth from the Sun.
Mastering these modes and understanding their effects in different materials and environments will enable you to solve problems involving heat movement and its impact on system performance. Knowledge of how these processes interact is fundamental for designing efficient systems that manage heat effectively.
Understanding the Laws of Thermodynamics
The foundational principles governing the behavior of energy in different systems are essential for solving problems related to heat, work, and energy flow. These rules establish how energy is conserved, transferred, and transformed. Understanding them allows for the prediction and analysis of energy interactions in various physical processes.
There are four key laws that outline how energy behaves in a closed system. The first law focuses on the conservation of energy, stating that energy can neither be created nor destroyed, only converted from one form to another. The second law introduces the concept of entropy, explaining that systems tend to move towards a state of greater disorder over time. The third law addresses the behavior of systems as they approach absolute zero temperature, where molecular motion ceases. The zeroth law sets the foundation for temperature measurement, stating that if two systems are in thermal equilibrium with a third, they are in equilibrium with each other.
By mastering these principles, you can better understand the constraints and possibilities of energy use in real-world systems, leading to more effective analysis and application of these ideas in practical scenarios.
Essential Equations for Thermodynamics Problems
Solving problems related to energy, heat, and work requires a solid understanding of the key mathematical relationships that govern these processes. Several fundamental equations are used to describe energy conversion, heat transfer, and other crucial concepts. Mastering these equations is critical for tackling complex scenarios and achieving accurate results.
First Law of Energy Conservation

The first law is expressed as:
ΔU = Q – W,
where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system. This equation helps in calculating energy changes during various processes and ensures the conservation of energy within a system.
Entropy Change and Second Law
The second law is often expressed in terms of entropy change:
ΔS = Q/T,
where ΔS is the change in entropy, Q is the heat transfer, and T is the temperature at which the transfer occurs. This equation is essential for understanding the direction of natural processes and the limitations of energy conversion efficiency.
Familiarity with these fundamental equations will allow you to solve a wide range of energy-related problems, applying the correct formulas to derive solutions and gain insights into system behavior.
How to Tackle Energy Conversion Questions
When approaching problems related to the transformation of energy, it’s important to first understand the process and the types of conversions involved. Energy can be transferred between various forms, such as heat to work or mechanical energy to electrical energy. The key to solving these problems lies in identifying the relevant laws and equations that govern these processes.
Start by carefully analyzing the system and the type of energy conversion it undergoes. Identify known quantities, such as the amount of heat supplied, the work done, or the efficiency of the system. From there, apply the appropriate equation, such as the first law of energy conservation, to calculate the desired result.
For example, in the case of heat engines, you might need to determine the work output based on the heat input and the efficiency of the engine. In such scenarios, it’s crucial to keep track of units and verify that all terms are properly accounted for to avoid errors in the calculations.
By breaking down the problem into smaller, manageable steps and systematically applying relevant principles, you’ll be able to effectively solve energy conversion-related problems with greater accuracy and confidence.
Thermodynamic Cycles and Their Importance
In energy systems, cycles play a central role in how energy is transferred and converted. These repeating processes help us understand how energy can be extracted, used, and restored in a system. Thermodynamic cycles form the backbone of many real-world applications, from power plants to refrigeration systems, making them critical to energy efficiency and performance.
Types of Cycles
There are several types of cycles commonly studied, including the Carnot cycle, Rankine cycle, and refrigeration cycles. Each has its own set of characteristics and applications, but all share the common goal of maximizing energy use and minimizing waste. For example, the Carnot cycle is often used as a theoretical model to determine the maximum efficiency of a heat engine.
Practical Applications

Understanding these cycles is essential for designing more efficient systems. By optimizing the various stages of a cycle, engineers can improve the performance of devices that rely on heat and energy conversion. From increasing the power output of engines to enhancing the cooling efficiency of refrigerators, knowledge of these cycles is key to advancing technology and reducing energy consumption.
In essence, thermodynamic cycles provide a framework for understanding how energy flows through a system and how that energy can be best utilized to perform work or generate useful output. Mastering these concepts is crucial for solving real-world challenges in energy systems.
Entropy and Its Role in Exams
Entropy is a central concept in understanding the behavior of energy within a system. It measures the degree of disorder or randomness, and it plays a crucial role in determining the efficiency of energy transformations. In various problem-solving scenarios, entropy helps explain why certain processes are irreversible and why energy disperses in a particular direction.
Key Points to Understand About Entropy
- Entropy as a Measure of Disorder: It quantifies the amount of disorder in a system, with higher values indicating more disordered states.
- Entropy Change in Processes: When energy is transferred or transformed, the entropy of the system typically increases. Understanding how to calculate this change is important for solving related problems.
- Second Law of Energy: Entropy is closely tied to the second law, which states that the total entropy of an isolated system always increases over time, driving processes toward greater disorder.
How to Approach Entropy-Based Problems
When faced with problems involving entropy, it is important to carefully identify the type of process being described–whether it is a reversible or irreversible transformation. Focus on the known variables, such as temperature and heat transfer, and apply the appropriate equations to calculate changes in entropy. This can help determine whether the process is efficient or if energy is being wasted due to increased disorder.
Mastering entropy calculations will not only deepen your understanding of energy flow but will also equip you with the tools to tackle complex problems related to the movement and transformation of energy in real-world systems.
Maximizing Efficiency in Thermodynamics Problems
When solving problems related to energy conversion and heat transfer, one of the key goals is to maximize efficiency. Efficient systems minimize energy loss and ensure that as much energy as possible is converted into useful work. Achieving this requires a solid understanding of the principles that govern energy flow and the constraints imposed by natural laws.
Strategies for Maximizing Efficiency
- Optimize Heat Transfer: Ensuring that heat is transferred efficiently can reduce losses. This involves selecting materials with high thermal conductivity and designing systems that minimize heat dissipation.
- Minimize Irreversibilities: Irreversible processes lead to higher energy losses. Striving for reversible processes, or those that approach reversibility, can significantly improve overall efficiency.
- Use Ideal Cycles: Studying and applying idealized cycles, such as the Carnot cycle, can provide a benchmark for maximum achievable efficiency, helping you understand how close real systems can come to optimal performance.
- Improve Insulation: Proper insulation prevents energy loss from heat exchange, helping systems retain energy and operate more efficiently.
Applying Efficiency Principles to Practical Problems
To maximize efficiency in real-world scenarios, it’s essential to carefully analyze the system’s components and identify areas where energy is being wasted. Whether you are working with engines, refrigerators, or any other energy-converting system, understanding the limitations and opportunities for improvement will allow you to solve problems more effectively and achieve better results.
By focusing on the key factors that influence energy transfer and conversion, you can approach problems with a mindset geared toward reducing waste and maximizing output, leading to more efficient and sustainable systems.
Common Mistakes in Thermodynamics Exams
When tackling problems related to energy transfer and transformation, it’s easy to fall into certain traps that can lead to incorrect solutions. These common errors often stem from misapplying fundamental concepts or overlooking key details. Recognizing these pitfalls can help you avoid them and improve your problem-solving accuracy.
One frequent mistake is failing to correctly apply the laws of energy conservation, which can result in incorrect calculations of work, heat, or efficiency. Another issue arises when assumptions about ideal conditions are made without considering real-world factors that may affect the system’s behavior. It’s also easy to mix up units or neglect to check dimensional consistency, leading to erroneous results.
Key Mistakes to Watch Out For
- Incorrect Application of the First Law: Often, students forget to account for all forms of energy entering or leaving a system, leading to imbalanced equations.
- Overlooking Assumptions in Ideal Processes: Not considering practical inefficiencies when working with theoretical models can skew results.
- Unit Conversion Errors: Failing to convert between units properly can lead to major discrepancies in final answers.
- Ignoring Entropy Changes: Not factoring in entropy changes in irreversible processes can result in misunderstandings of system behavior.
Avoiding Common Pitfalls
To avoid these mistakes, take the time to carefully read through the problem, identify all known variables, and double-check each step of your calculations. Remember that understanding the underlying principles and recognizing the assumptions of each system will help you apply the correct equations and reasoning. By staying organized and mindful of common errors, you can enhance your ability to solve complex problems with precision.
Strategies for Solving Complex Problems
Solving intricate problems that involve energy transformation and material properties requires a structured approach. By breaking down the problem into manageable steps and applying fundamental principles systematically, you can simplify even the most complex scenarios. A methodical approach not only helps in identifying the correct solution but also minimizes errors and ensures that no critical detail is overlooked.
Approach to Problem Solving
- Understand the Problem Fully: Begin by carefully reading the problem and identifying all the given data and the unknowns. Ensure you have a clear understanding of the conditions and constraints involved.
- Identify Relevant Principles: Once the problem is understood, identify which physical laws or concepts are applicable. This may include energy conservation, entropy, or specific heat capacities depending on the context.
- Break Down the Problem: Divide the complex problem into smaller, simpler parts. This allows you to solve each component separately before combining the results for the final answer.
- Check Units and Dimensions: Ensure that all units are consistent throughout the problem. Mistakes often arise from unit conversion errors, so always verify dimensions as you work through the steps.
- Use Idealized Models First: Start by solving using ideal conditions (e.g., assuming reversible processes), which can provide an upper limit or benchmark for performance. Then, adjust for real-world factors if necessary.
Steps to Verify Solutions
- Double-Check Calculations: After solving, revisit each step of the calculation to ensure no errors were made. If time allows, use an alternative method to verify the result.
- Consider Physical Realism: After obtaining a solution, evaluate whether the result makes sense in the context of real-world systems. If the result seems unrealistic, re-examine your assumptions.
- Practice Problem Solving: The more problems you solve, the more familiar you become with the patterns and techniques that work best. Regular practice builds confidence and sharpens problem-solving skills.
By approaching complex problems methodically and checking your results at each stage, you will improve your ability to navigate challenges and arrive at accurate solutions more efficiently.
Practical Applications of Thermodynamics
Understanding the principles of energy flow and transformation is essential for solving real-world problems across various industries. From power generation to refrigeration, these concepts play a crucial role in improving efficiency and designing systems that are both sustainable and cost-effective. By applying these principles, engineers and scientists are able to optimize processes and create innovative solutions to modern challenges.
One of the most prominent applications is in power plants, where the conversion of heat into work is central to generating electricity. The efficiency of these systems, such as steam engines or gas turbines, depends on the careful management of energy and heat loss. Similarly, refrigeration and air conditioning systems rely on controlling temperature and pressure to transfer heat effectively, providing comfort and preserving food and other goods.
Energy Systems and Power Generation

- Heat Engines: Heat engines are used to convert thermal energy into mechanical work, with applications in power generation and transportation, such as in internal combustion engines and steam turbines.
- Combined Cycle Power Plants: These plants use both gas and steam turbines in tandem to increase efficiency, maximizing energy extraction from fuel while minimizing waste.
- Geothermal Energy: Geothermal power plants harness heat from the Earth’s core to generate electricity, providing a sustainable and renewable energy source.
Cooling Systems and Refrigeration
- Refrigerators and Air Conditioners: These systems rely on refrigerants and controlled thermodynamic cycles to remove heat from a confined space, ensuring temperature control in homes, buildings, and vehicles.
- Heat Pumps: Heat pumps transfer heat from one location to another and are used for both heating and cooling, offering an energy-efficient alternative to traditional systems.
In addition to energy generation and temperature control, these principles also find applications in fields such as materials science, environmental protection, and even food processing. Their broad applicability highlights their importance in creating more efficient, sustainable, and practical solutions to the challenges of modern society.
Thermodynamics in Real-World Scenarios

The principles of energy transformation and heat management are not just theoretical concepts but are actively applied in various real-life settings. From everyday appliances to industrial machinery, understanding how energy flows and changes form is key to improving efficiency and solving complex problems. These concepts are crucial in numerous fields, helping to shape innovations and optimize performance in diverse systems.
For example, the operation of cars, airplanes, and industrial engines all rely heavily on the conversion of heat into useful work. Engineers utilize these principles to design vehicles and machinery that maximize fuel efficiency, minimize waste, and reduce environmental impact. In the energy sector, power plants convert heat into electricity, and efforts are continuously being made to enhance these systems for better performance and sustainability.
Energy Conversion in Power Generation

- Steam Turbines: Steam is used to generate mechanical work by expanding through turbines, which are a key component in power plants. The efficiency of these systems determines how much electricity can be generated from fuel.
- Combined Heat and Power Systems: These systems use waste heat from electricity generation to provide heating, ensuring minimal energy loss and enhancing overall system efficiency.
- Renewable Energy Systems: Solar, wind, and hydroelectric plants convert natural resources into electricity, relying on efficient energy transfer mechanisms to maximize output and minimize costs.
Applications in Everyday Life
- Home Heating and Cooling: Air conditioning and heating systems in homes and offices rely on controlled energy transfer to maintain comfort, with innovations constantly improving their energy efficiency.
- Refrigeration: From refrigerators to freezers, these devices use cycles to transfer heat from inside to the external environment, preserving food and other items by keeping them at low temperatures.
- Automotive Engines: Modern cars are designed to maximize fuel efficiency, with innovations like hybrid engines that optimize energy usage through better heat conversion processes.
These are just a few examples of how energy transfer principles impact our daily lives. The continuous development and refinement of energy systems are vital for addressing global challenges such as sustainability, efficiency, and reducing carbon footprints, making this field essential in both practical applications and future advancements.
Types of Questions in Thermodynamics Exams
In assessments related to energy transfer and heat systems, the focus is often placed on a variety of problem-solving scenarios that test understanding of core concepts. These challenges range from theoretical calculations to practical applications, designed to evaluate both comprehension and the ability to apply knowledge in real-world contexts. Typically, they require learners to manipulate equations, analyze systems, and draw conclusions based on given conditions.
The questions can vary widely, ranging from simple multiple-choice formats to more complex, open-ended problems that require detailed solutions. Below is a breakdown of some common types of challenges encountered in these evaluations:
| Type of Problem | Description | Example Focus |
|---|---|---|
| Conceptual Understanding | These questions assess foundational knowledge and comprehension of principles related to energy and heat. | Understanding the relationship between work and heat, definitions of energy conservation |
| Problem-Solving Calculations | These require applying mathematical formulas to solve numerical problems, often with real-world contexts. | Calculating work done in a closed system, determining efficiency of heat engines |
| Systems Analysis | Involve analyzing different types of systems, such as closed, open, or isolated, and calculating the effects of energy flow. | Evaluating heat transfer in various systems, finding energy changes in a cycle |
| Cycle Analysis | These questions focus on analyzing processes that occur over a complete cycle, such as a Carnot or Rankine cycle. | Efficiency of heat engines, work produced in cyclic processes |
| Application-Based Problems | These questions focus on applying theoretical principles to practical scenarios, such as in engineering or daily applications. | Energy efficiency in industrial machinery, heat pump operation |
By addressing these various types of problems, assessments challenge learners to demonstrate a comprehensive understanding of how energy behaves in different contexts, both theoretically and practically. Each question type serves a unique purpose in evaluating different aspects of knowledge and problem-solving skills.
Tips for Last-Minute Exam Preparation
When time is running short and the clock is ticking down, it’s important to focus on effective strategies that can help you maximize your efforts. Instead of trying to cover everything, concentrating on the most critical areas can make a significant difference in your performance. By organizing your time wisely, reviewing key concepts, and practicing with real-world problems, you can boost your confidence and improve your results.
Focus on High-Impact Areas
At the last minute, it’s essential to prioritize topics that are both challenging and frequently tested. Reviewing key principles, understanding the most common problem types, and revisiting your notes on complex concepts will help solidify your understanding. Consider these high-priority areas:
| Topic | Reason for Importance | Quick Study Tip |
|---|---|---|
| Core Principles | They form the foundation of most problems. | Review summaries and flashcards to reinforce definitions. |
| Common Calculations | They often appear in various forms. | Practice problems that involve key formulas and conversions. |
| Systems Analysis | Understanding how different systems operate is crucial. | Go over examples of energy transfers and system interactions. |
| Efficiency and Work | These topics are integral to many real-world applications. | Focus on understanding how to compute and interpret efficiency. |
Practice with Previous Scenarios
Another effective method for last-minute preparation is to review past materials. Whether it’s previous tests, sample problems, or practice exercises, revisiting old problems can reveal patterns in how questions are framed and what kind of solutions are expected. This practice can also help you manage your time more effectively during the actual assessment.
By focusing on these essential topics and engaging in targeted practice, you can approach your preparation in a more focused and strategic way, even in the final hours before the deadline.