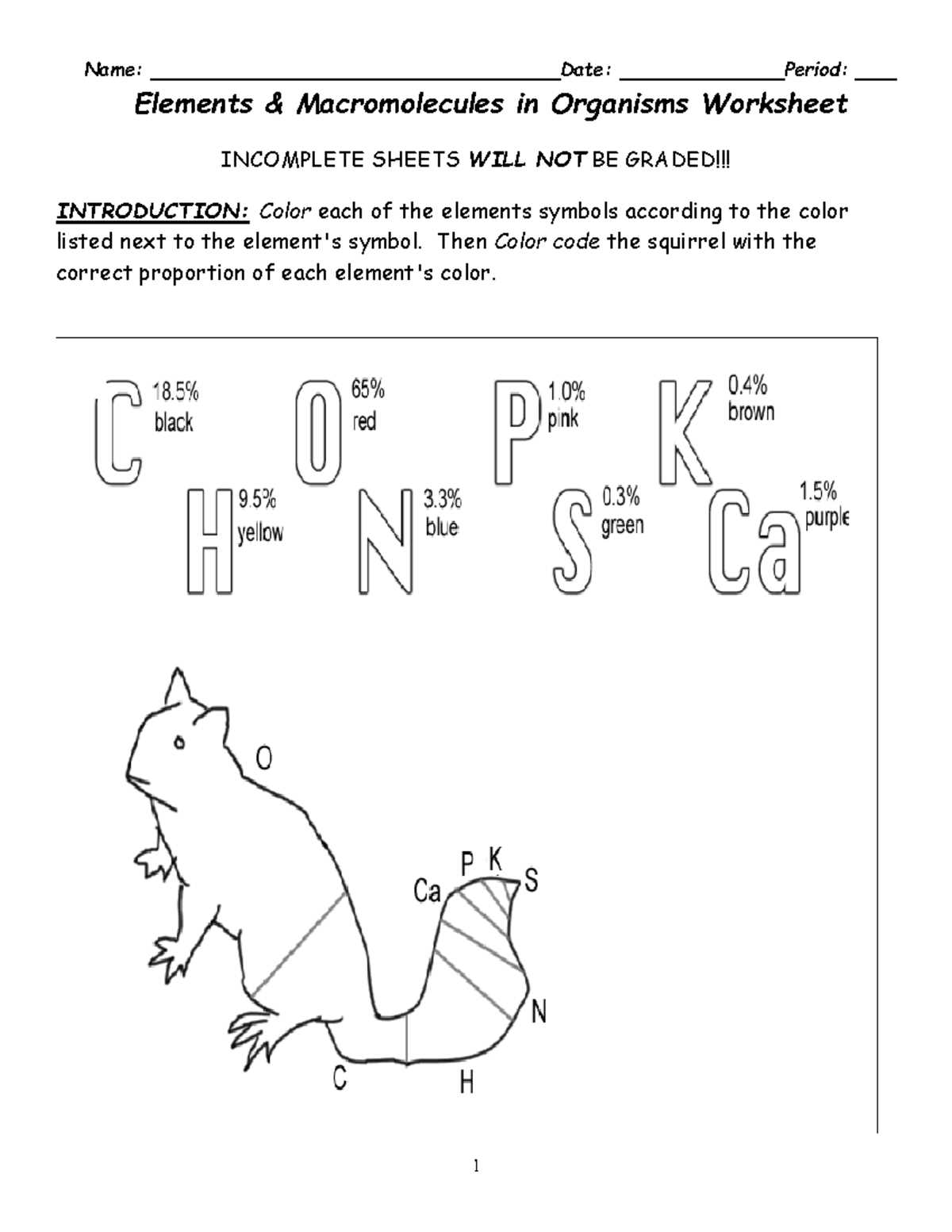
Life is built upon a variety of essential substances that serve as the foundation for all living systems. These fundamental building blocks are involved in countless processes, enabling growth, energy transfer, and cellular maintenance. They are crucial for sustaining life and ensuring the proper functioning of biological structures.
Carbon plays a central role in the formation of complex molecules that power vital processes, while proteins, lipids, and nucleic acids are involved in everything from energy storage to genetic expression. Each of these key components interacts in a highly organized manner to create life-sustaining functions within cells.
This section will explore the role of various substances and large molecular structures in the life cycle, their interactions, and their significance to biological processes. It is essential to understand how these substances work together to maintain life, health, and functionality across all living systems.
Elements and Macromolecules in Organisms Worksheet Answers
Understanding the building blocks of life is essential for comprehending the vast complexity of biological systems. These basic components combine in various ways to form complex structures that carry out the processes needed to sustain life. This section addresses common topics related to how these components function, their interactions, and the processes they enable within living entities.
The following are key concepts that illustrate the roles of these substances in maintaining life:
- Carbon is a crucial component, forming the backbone of most large molecules.
- Proteins serve as enzymes, structural units, and facilitate metabolic reactions.
- Lipids are vital for energy storage, membrane structure, and insulation.
- Nucleic acids store and transmit genetic information.
- Water is essential for regulating temperature and facilitating chemical reactions.
Each of these substances plays a vital role in ensuring cellular function and overall health. For example, proteins are involved in catalyzing reactions, while lipids form barriers that protect cells. Nucleic acids, on the other hand, carry genetic information that directs cellular processes. Understanding how these components work together helps explain the complexity of life itself.
To gain a better understanding of these topics, consider how each of these components is involved in specific processes such as energy production, genetic inheritance, and cell communication. The interactions between them are essential for life’s continuity and adaptability.
Overview of Key Biological Elements
The fundamental substances that support life are crucial for the structure and function of all living systems. These basic components are involved in nearly every biological process, from providing energy to enabling growth and reproduction. Understanding these substances is essential for exploring how life functions at a molecular level.
There are several key substances that play a critical role in supporting cellular activities. These include basic materials such as carbon, hydrogen, oxygen, nitrogen, and other essential compounds. Together, they form the building blocks of larger, more complex molecules that are vital for the survival of living systems.
| Substance | Role in Biological Systems |
|---|---|
| Carbon | Forms the backbone of organic molecules, critical for structure and energy storage. |
| Hydrogen | Part of water and many organic compounds, essential for energy reactions. |
| Oxygen | Crucial for cellular respiration and energy production in cells. |
| Nitrogen | Integral to amino acids and proteins, necessary for cellular function and growth. |
| Phosphorus | Key component of nucleic acids, important for energy transfer in cells. |
| Sulfur | Found in some amino acids and proteins, plays a role in metabolic processes. |
Each of these substances plays a specific role that contributes to the overall functioning of cells and systems. Without these building blocks, essential processes such as energy production, growth, and reproduction could not occur. The proper balance and interaction between these materials ensure the survival and adaptability of life in a variety of environments.
Importance of Carbon in Life Forms

Carbon plays a fundamental role in supporting the structure and function of all living entities. Its unique properties allow it to form diverse and complex molecules essential for biological processes. The ability of carbon to bond with various other atoms creates the foundation for the molecules that drive energy production, cellular growth, and genetic inheritance.
Without this versatile element, life would not be able to exist as we know it. Carbon’s ability to form stable yet flexible structures enables the creation of long chains and rings, which are the building blocks of crucial biomolecules. Here are some key ways carbon is utilized:
- Energy storage: Carbohydrates and lipids, both based on carbon, store energy that cells can later use for various activities.
- Structural integrity: Carbon atoms are integral to the construction of vital cellular structures, including membranes and the framework of proteins.
- Genetic information: Carbon-rich nucleic acids, such as DNA and RNA, are responsible for storing and transferring genetic blueprints.
- Catalysis: Many enzymes, essential for biochemical reactions, are made from carbon-containing molecules.
The role of carbon is not limited to providing structure; it is also essential for regulating life processes through the formation of molecules that enable communication, energy flow, and adaptation. This makes it the backbone of all biological systems on Earth.
What Are Macromolecules in Biology
In the realm of biology, large, complex molecules play essential roles in the structure and function of living systems. These massive compounds are responsible for carrying out a variety of tasks necessary for growth, energy storage, and cellular communication. Their size and intricate structures enable them to perform highly specific functions within cells and tissues.
These large compounds are primarily made up of smaller units that link together in repeating patterns, creating long chains or networks. The diversity of these molecules is a result of the various ways in which smaller units can combine and interact. Some of the most important biological compounds include:
- Proteins: Comprised of amino acids, these perform tasks such as catalyzing chemical reactions, supporting cell structure, and defending the body against pathogens.
- Carbohydrates: Sugars and starches provide energy storage and structural support in cells, particularly in plants.
- Lipids: These molecules store energy and form protective barriers around cells, such as membranes.
- Nucleic acids: DNA and RNA are essential for storing genetic information and directing protein synthesis.
Each of these large compounds serves as a cornerstone of life, providing the necessary tools for cells to function, reproduce, and respond to their environment. Their intricate design allows them to carry out specialized tasks, making them indispensable to the complexity of living systems.
Types of Carbohydrates and Their Functions
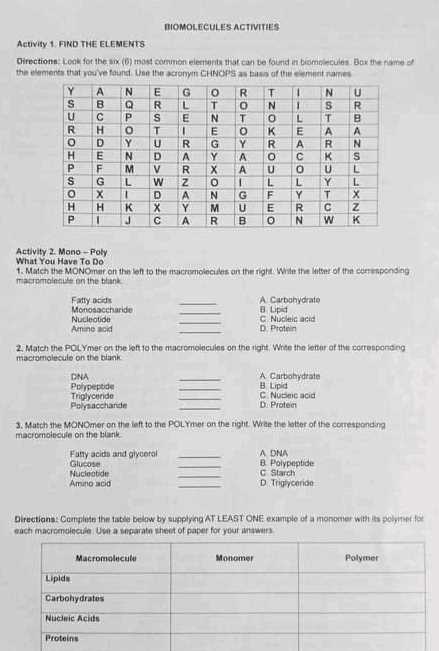
Carbohydrates are essential for providing energy and structural support within cells. These compounds come in various forms, each serving a distinct role in the body. They are composed primarily of carbon, hydrogen, and oxygen, and are critical for fueling cellular processes, storing energy, and supporting tissue structure.
Simple Sugars
Simple carbohydrates, or sugars, are the most basic form of this nutrient. These are quickly absorbed by the body to provide immediate energy. They can be found in foods like fruits, honey, and some vegetables. Simple sugars consist of either monosaccharides, like glucose, or disaccharides, such as sucrose.
- Glucose: A primary source of energy for cells, especially in the brain and muscles.
- Fructose: Found in fruits, it is converted to glucose in the body for use as energy.
- Sucrose: Common table sugar, composed of glucose and fructose, used in food and beverages.
Complex Carbohydrates
Complex carbohydrates consist of multiple sugar molecules linked together in long chains. These provide sustained energy and are slower to digest, helping maintain steady blood sugar levels. Found in foods like whole grains, potatoes, and legumes, complex carbohydrates are also important for fiber content, which aids digestion.
- Starch: A primary energy source for many plants, it is found in foods like rice, potatoes, and corn.
- Cellulose: A structural component in plants, it provides support to plant cells and is also a source of dietary fiber in the human diet.
- Glycogen: Stored in muscles and the liver, it acts as a short-term energy reserve in animals.
Each type of carbohydrate plays a crucial role in supporting the body’s functions, whether by providing quick bursts of energy or contributing to long-term storage and structural integrity. Understanding these carbohydrates is vital for managing diet and maintaining healthy metabolic processes.
Proteins and Their Biological Roles
Proteins are central to nearly all processes in living systems, serving as the workhorses of the cell. These complex molecules are involved in a wide range of essential functions, from catalyzing chemical reactions to supporting cellular structure. Their diverse roles are critical for maintaining life, as they are responsible for building, repairing, and maintaining tissues and organs.
Comprised of amino acids, proteins can take on various shapes and structures, which directly relate to their function. The sequence of amino acids determines how a protein folds and what specific task it performs. Below are some of the primary functions of proteins in biological systems:
- Enzymes: Proteins that speed up biochemical reactions, allowing cells to carry out essential processes such as digestion and metabolism.
- Structural Support: Proteins like collagen and keratin provide structural integrity to tissues such as skin, bones, and hair.
- Transport: Proteins like hemoglobin transport oxygen through the blood, while others move molecules across cell membranes.
- Immune Response: Antibodies are proteins that help defend the body against pathogens, playing a key role in immunity.
- Cell Signaling: Many proteins act as receptors or messengers, helping cells respond to external signals and regulate internal processes.
The vast array of tasks that proteins perform makes them indispensable for proper cellular function and overall health. Without these versatile molecules, life would not be able to sustain itself or adapt to changing environments.
Proteins and Their Biological Roles
Proteins are central to nearly all processes in living systems, serving as the workhorses of the cell. These complex molecules are involved in a wide range of essential functions, from catalyzing chemical reactions to supporting cellular structure. Their diverse roles are critical for maintaining life, as they are responsible for building, repairing, and maintaining tissues and organs.
Comprised of amino acids, proteins can take on various shapes and structures, which directly relate to their function. The sequence of amino acids determines how a protein folds and what specific task it performs. Below are some of the primary functions of proteins in biological systems:
- Enzymes: Proteins that speed up biochemical reactions, allowing cells to carry out essential processes such as digestion and metabolism.
- Structural Support: Proteins like collagen and keratin provide structural integrity to tissues such as skin, bones, and hair.
- Transport: Proteins like hemoglobin transport oxygen through the blood, while others move molecules across cell membranes.
- Immune Response: Antibodies are proteins that help defend the body against pathogens, playing a key role in immunity.
- Cell Signaling: Many proteins act as receptors or messengers, helping cells respond to external signals and regulate internal processes.
The vast array of tasks that proteins perform makes them indispensable for proper cellular function and overall health. Without these versatile molecules, life would not be able to sustain itself or adapt to changing environments.
Understanding Lipids and Their Functions
Lipids are essential compounds that play a crucial role in maintaining the structure and function of cells. These hydrophobic molecules are not only involved in energy storage but also contribute to the formation of cell membranes, insulation, and protection. Unlike other nutrients, lipids are diverse in their structure and function, making them vital for numerous physiological processes.
There are various types of lipids, each serving a unique purpose in the body. Some act as long-term energy reserves, while others are integral components of cellular membranes or serve as signaling molecules. Below are some of the key roles that lipids play in living systems:
- Energy Storage: Lipids, particularly triglycerides, store energy efficiently, providing a dense source of fuel for the body.
- Cell Membrane Structure: Phospholipids form the bilayer of cell membranes, creating a barrier that regulates the movement of substances into and out of the cell.
- Insulation and Protection: Lipids help insulate organs, regulate body temperature, and provide cushioning to protect vital organs from physical damage.
- Hormone Production: Certain lipids, such as sterols, are precursors to hormones like steroids, which regulate various bodily functions.
- Cell Communication: Lipid-based molecules, such as eicosanoids, are involved in signaling pathways that help coordinate cellular responses to external stimuli.
Due to their diverse roles, lipids are indispensable for maintaining homeostasis, energy balance, and the proper functioning of cells and organs. Without lipids, life would be unable to maintain its structural integrity, adapt to changes, or efficiently store and use energy.
How Water Supports Life Functions
Water is a vital substance that sustains countless processes essential for life. Its unique properties make it indispensable for maintaining cellular activities, regulating temperature, and facilitating chemical reactions. Without this compound, life as we know it would not be possible, as it supports numerous functions critical to the survival and stability of living systems.
Water’s ability to dissolve a wide range of substances allows it to act as a medium for nutrient transport, waste removal, and the regulation of internal conditions. Here are some key roles that water plays in supporting life functions:
- Temperature Regulation: Water’s high heat capacity helps maintain a stable temperature within cells and organisms, preventing extreme fluctuations that could damage biological structures.
- Solvent for Chemical Reactions: Water dissolves polar substances, allowing for the proper function of enzymes and metabolic pathways crucial for growth and energy production.
- Transport of Nutrients: Water is a primary component of blood and other bodily fluids, enabling the movement of nutrients, gases, and waste products throughout an organism.
- Lubrication and Cushioning: Water serves as a lubricant in joints and tissues, reducing friction and protecting organs from mechanical stress.
- Cellular Structure and Function: Water maintains cell turgidity, ensuring cells retain their shape and enabling the proper functioning of cellular machinery.
In summary, water is not just a simple liquid but a cornerstone of life. Its ability to participate in diverse physiological functions makes it essential for the growth, maintenance, and overall health of living beings.
The Role of Enzymes in Metabolism
Enzymes are specialized proteins that act as catalysts, speeding up chemical reactions within cells. They play a critical role in metabolism, facilitating the breakdown of nutrients and the synthesis of essential compounds. Without enzymes, these processes would occur too slowly to sustain life. By lowering the energy required for reactions, enzymes enable cells to maintain proper function and energy balance.
Metabolic reactions are divided into two main categories: catabolic, where larger molecules are broken down into smaller ones, and anabolic, where smaller molecules are assembled into larger, more complex structures. Enzymes are involved in both types of reactions, ensuring that they proceed efficiently and at the right pace.
How Enzymes Function in Metabolism
Enzymes bind to specific substrates, forming an enzyme-substrate complex. This complex facilitates the transformation of substrates into products by reducing the activation energy needed for the reaction. The specificity of enzymes ensures that each metabolic pathway is carefully regulated and carried out only when needed.
Examples of Enzyme-Driven Metabolic Pathways

Different enzymes control various metabolic pathways, such as the digestion of carbohydrates or the synthesis of proteins. Below is a table highlighting some common metabolic reactions and the enzymes involved:
| Reaction Type | Enzyme | Function |
|---|---|---|
| Breakdown of Glucose | Hexokinase | Phosphorylates glucose to start glycolysis |
| Protein Synthesis | Ribosome | Assembles amino acids into polypeptide chains |
| Fat Breakdown | Lipase | Hydrolyzes triglycerides into fatty acids and glycerol |
In summary, enzymes are essential to metabolism, ensuring that chemical reactions occur rapidly, efficiently, and in a controlled manner. Their specific action allows cells to maintain the balance necessary for life.
Building Blocks of Amino Acids
Amino acids are the fundamental units that form proteins, playing a vital role in the structure, function, and regulation of the body’s tissues and organs. Each amino acid is composed of a central carbon atom, bonded to an amino group, a carboxyl group, a hydrogen atom, and a distinctive side chain, or R-group, that determines its unique properties. These building blocks link together in specific sequences to create proteins, each with a unique shape and function.
The R-group, or side chain, is what makes each amino acid distinct. It can vary in size, charge, and polarity, giving proteins their diversity in function. The way amino acids interact with one another, based on their side chains, influences the protein’s three-dimensional structure and its ability to perform its biological role.
Essential vs. Non-Essential Amino Acids
Amino acids are classified into two main categories based on how they are obtained:
- Essential Amino Acids: These cannot be synthesized by the body and must be obtained through the diet. Examples include leucine, lysine, and valine.
- Non-Essential Amino Acids: The body can synthesize these amino acids, so they do not need to be consumed directly. Examples include alanine, asparagine, and glutamine.
The Peptide Bond and Protein Formation

When amino acids join together, they form peptide bonds through a dehydration reaction, resulting in the formation of peptides and proteins. These peptide bonds create long chains of amino acids, which then fold into complex shapes. The sequence of amino acids within a peptide chain determines the protein’s structure and its biological function, making the arrangement of these building blocks crucial for the activity of life.
Types of Bonds in Organic Molecules
The structure and function of complex compounds are determined by the types of connections between their atoms. These connections, or bonds, play a crucial role in the stability and reactivity of the molecules. In organic compounds, there are three primary types of bonds: covalent, ionic, and hydrogen bonds. Each of these has unique characteristics and contributes differently to the properties of the molecule.
Covalent bonds are the most common type found in biological systems, where atoms share electrons to form stable connections. Ionic bonds occur when one atom transfers electrons to another, creating charged particles that attract each other. Hydrogen bonds, though weaker, are essential for the structure of many biomolecules, particularly in water and DNA, where they help maintain stability and facilitate interactions between molecules.
Covalent Bonds

Covalent bonds form when atoms share pairs of electrons, resulting in the creation of a stable molecule. This type of bond is commonly seen in organic compounds such as carbohydrates, proteins, and lipids. The strength of covalent bonds makes them crucial for maintaining the integrity of cellular structures.
Ionic Bonds
Ionic bonds occur when one atom gives up one or more electrons to another, resulting in the formation of positively and negatively charged ions. These oppositely charged ions attract each other, forming a bond. Ionic interactions are important in many biochemical processes, including the formation of salts and the transport of charged particles across cell membranes.
Hydrogen Bonds
Hydrogen bonds are weak interactions that occur when a hydrogen atom covalently bonded to an electronegative atom, like oxygen or nitrogen, is attracted to another electronegative atom. While these bonds are weaker than covalent and ionic bonds, they are vital for the structure of DNA, proteins, and the properties of water, such as its high boiling point and solvent capabilities.
Structure of DNA and RNA Molecules
The genetic material within living organisms is stored and transmitted by two key types of molecules: one involved in long-term storage of genetic information, and the other in the transmission of this information during processes such as protein synthesis. These molecules have unique structures that enable them to perform their specific roles within cells. Though both share some similarities, each has distinct features that allow them to serve their individual functions effectively.
The first molecule is a double-stranded helix, where two strands twist around each other, forming a stable structure capable of holding vast amounts of genetic data. The second molecule, though structurally simpler, plays a vital role in transferring genetic information from the nucleus to the site of protein production. Both molecules are made up of smaller subunits, which contribute to their complexity and functionality.
DNA Structure
DNA (deoxyribonucleic acid) consists of two long chains of nucleotides twisted into a double helix. Each nucleotide is composed of a sugar molecule, a phosphate group, and one of four nitrogenous bases: adenine (A), thymine (T), cytosine (C), and guanine (G). The two strands are held together by hydrogen bonds between complementary base pairs: adenine pairs with thymine, and cytosine pairs with guanine. The sequence of these bases encodes genetic information, which is used in cellular processes like replication and transcription.
RNA Structure
RNA (ribonucleic acid) shares similarities with DNA but differs in several key aspects. It is usually single-stranded and contains the sugar ribose, unlike DNA’s deoxyribose. RNA also has uracil (U) instead of thymine (T) as one of its nitrogenous bases. In RNA, adenine pairs with uracil, while cytosine pairs with guanine. RNA plays a crucial role in translating genetic instructions into functional proteins, particularly through its messenger form (mRNA), which carries the code from DNA to ribosomes for protein synthesis.
Interaction Between Elements and Macromolecules
The interactions that occur between small chemical components and larger molecular structures are essential for the proper functioning of living systems. These smaller building blocks, when combined in specific arrangements, form larger, complex structures that carry out a wide variety of biological functions. The way these structures are assembled and interact with each other directly influences the biological processes necessary for life.
A key aspect of this interaction is how certain smaller molecules provide the foundation for the larger compounds, offering stability and the ability to perform specific tasks within cells. These processes are highly regulated, ensuring that the correct molecular structures are formed at the right time and place to maintain cellular integrity and efficiency.
Key Interactions
| Small Molecule | Large Molecule | Role in Interaction |
|---|---|---|
| Carbon | Proteins | Forms backbone structures that support enzymatic activity |
| Hydrogen | DNA | Stabilizes double helix structure |
| Oxygen | Lipids | Contributes to energy storage and cellular membranes |
| Nitrogen | RNA | Participates in genetic information transfer |
Dynamic Role of Chemical Bonds
The interactions between small molecules and larger compounds often rely on various types of chemical bonds, including covalent, ionic, and hydrogen bonds. These bonds facilitate the structural integrity and functionality of the larger compounds. For example, covalent bonds help maintain the stability of complex structures, while hydrogen bonds play a key role in maintaining the shape of proteins and nucleic acids, ensuring their proper function within the cell.
How Nutrients Are Transported in Cells
The efficient movement of essential substances within cells is critical for maintaining cellular functions and overall health. This process involves a range of mechanisms that allow vital compounds, such as sugars, amino acids, and ions, to enter and exit the cell, ensuring that the cell has the necessary components for energy production, growth, and repair. The transport of nutrients is tightly regulated and often occurs through specialized structures within the cell membrane.
There are several key methods by which nutrients are moved across the cell membrane:
- Passive Transport: This process does not require energy and relies on the natural movement of molecules from areas of high concentration to areas of low concentration. Diffusion and osmosis are common examples.
- Active Transport: This mechanism requires energy to move substances against their concentration gradient, often using transport proteins to facilitate the process.
- Endocytosis: A form of bulk transport where the cell membrane engulfs large molecules or particles, bringing them into the cell in vesicles.
- Exocytosis: The reverse of endocytosis, this process involves the expulsion of substances from the cell, such as waste or secreted proteins, through vesicles fusing with the cell membrane.
Each of these methods plays a unique role in ensuring that nutrients are effectively absorbed, distributed, and utilized by the cell. Understanding these transport mechanisms is crucial for comprehending how cells interact with their environment and maintain their internal balance.
Energy Storage in Living Systems
Storing energy for future use is essential for the proper functioning of living systems. This process ensures that vital functions, such as growth, movement, and reproduction, can continue even when external energy sources are not readily available. Various compounds and structures play key roles in the storage, release, and regulation of energy within cells and tissues.
Types of Energy Storage
Living systems primarily rely on two forms of energy storage:
- Short-term Storage: The most common form is stored in the form of simple sugars, such as glucose, which can be rapidly utilized by cells to meet immediate energy demands.
- Long-term Storage: For more extended energy needs, systems store energy in more complex forms, such as fats and starches, which can be broken down when necessary over a longer period.
Storage Mechanisms
Energy is stored in various ways within cells, using specialized molecules and structures:
- Glycogen: This is a polysaccharide used by animals to store energy, mainly in liver and muscle cells. It can be quickly converted back into glucose when needed.
- Fat Cells: In many living systems, fat cells act as long-term energy reserves. Triglycerides stored in adipose tissue are broken down into fatty acids when the body requires extra energy.
- Starch: Plants primarily store energy in the form of starch, which is found in roots, stems, and seeds. Starch can be converted into glucose when the plant requires energy.
By maintaining these energy reserves, living systems ensure they have a continuous supply of power for vital processes, adapting to fluctuating environmental conditions and energy availability.
Impact of Deficiencies in Biological Molecules
A lack of essential compounds in living systems can lead to a variety of health issues and impair normal biological functions. When key substances are insufficient or absent, the body’s processes can be disrupted, affecting everything from cellular activity to overall system stability. Deficiencies can occur for a number of reasons, including poor diet, absorption issues, or metabolic dysfunctions.
Consequences of Deficiency
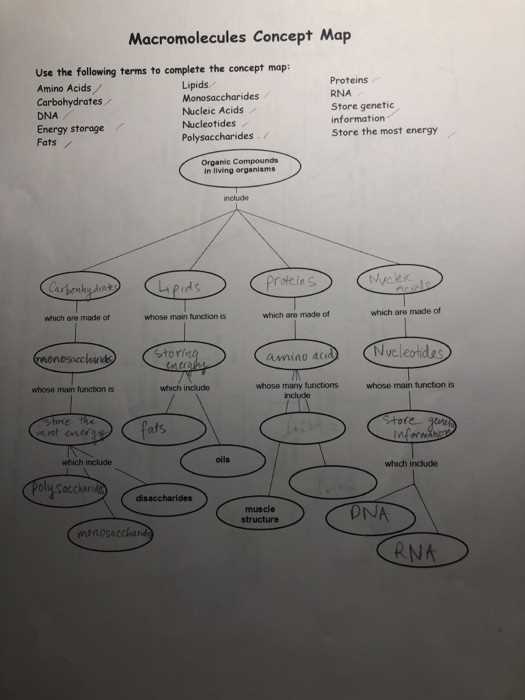
Deficiencies in vital compounds can result in a range of symptoms and health conditions:
- Energy Imbalance: A shortage of energy-rich compounds like carbohydrates or fats can lead to fatigue, muscle weakness, and a decreased ability to perform basic tasks.
- Weakened Immune Response: Insufficient amounts of certain proteins and vitamins can impair the immune system, making the body more vulnerable to infections.
- Growth Retardation: Lack of necessary building blocks, such as amino acids or nucleotides, can stunt growth and delay the development of cells and tissues.
- Organ Dysfunction: Deficiencies in structural compounds like lipids or proteins may cause organ malfunctions, impacting functions like digestion or circulation.
Common Deficient Compounds
Some of the most commonly deficient substances include:
- Vitamins: These organic compounds are crucial for enzyme function and metabolic processes. A lack of vitamins such as vitamin C or D can lead to conditions like scurvy or rickets.
- Minerals: Essential inorganic nutrients like iron, calcium, and magnesium are required for bone strength, oxygen transport, and cellular signaling. Their deficiency can lead to anemia, osteoporosis, and other disorders.
- Proteins: Inadequate protein intake can cause muscle loss, impaired immune function, and delayed tissue repair.
- Fatty Acids: Deficient essential fatty acids can affect cell membrane integrity, leading to skin issues, hormonal imbalances, and neurological problems.
Addressing these deficiencies is critical for maintaining health and ensuring the proper functioning of biological systems. By ensuring a balanced intake of nutrients, the body can better support its various physiological processes.
Summary of Worksheet Concepts
This section consolidates the key ideas discussed in the previous sections, focusing on the core principles related to the building blocks of life and their functions. A deeper understanding of how basic substances interact, form complex structures, and contribute to biological processes is essential for grasping the intricate mechanisms that sustain life.
Main Ideas Covered
- Structural Foundations: The fundamental units that make up living systems include various compounds that contribute to structure, function, and stability.
- Energy Dynamics: Different substances provide energy storage, release, and conversion, supporting growth, movement, and other cellular activities.
- Functional Roles: Each compound plays a specific role in processes such as metabolism, immune defense, and cellular communication.
- Transport Mechanisms: Key components are moved within cells and across membranes, ensuring the proper functioning of all systems.
Important Connections

- Interdependence: The substances discussed rely on each other to maintain homeostasis, as a deficiency in one can affect the function of others.
- Dynamic Interactions: The interactions between different types of compounds are vital for life, allowing for adaptations and responses to environmental changes.
- Continuous Exchange: Nutrient uptake, waste removal, and energy production are ongoing processes that are closely tied to the availability and balance of specific substances.
By understanding these concepts, we can better appreciate the complexity of life and the various processes that allow it to thrive.