The study of matter and its fundamental building blocks has fascinated scientists for centuries. In this section, we explore the foundational principles that shape our understanding of the microscopic world. By breaking down complex ideas into simpler concepts, we uncover how the smallest components of matter interact and form the universe around us.
From the early models that attempted to describe the structure of matter to modern interpretations based on experimental evidence, we trace the evolution of scientific thought. This journey not only highlights key discoveries but also clarifies the essential principles that govern the behavior of particles within substances.
As we examine the key concepts, we will focus on the essential features of matter, including the nature of its constituents and their interactions. A solid grasp of these concepts lays the foundation for further exploration into chemistry, physics, and the broader scientific landscape.
Atomic Theory Worksheet Answers
In this section, we will explore key concepts related to the structure of matter and its fundamental components. By delving into the basic principles and providing clear explanations, we aim to clarify the relationships between particles and the forces that govern their interactions. Understanding these concepts is essential for building a strong foundation in the physical sciences.
Key Principles of Matter’s Structure
The building blocks of all substances are particles that come together to form various types of matter. These particles are governed by specific laws that define their behavior, including how they bond with one another and interact in different environments. By examining these building blocks, we can better understand the overall structure of matter and how it influences the properties of substances.
Interactions and Behavior of Subatomic Particles
Subatomic particles, such as electrons, protons, and neutrons, play a central role in the behavior of matter. Each particle has its own characteristics that contribute to the overall structure of the atom and determine its chemical properties. By analyzing their interactions, we gain insight into how atoms combine to form molecules and how different types of matter react under various conditions.
Overview of Atomic Structure
Understanding the composition of matter begins with examining its most fundamental units. These tiny building blocks form the foundation of all substances and determine their properties. At the core of this structure are particles that come together to create different forms of matter, influencing everything from chemical reactions to physical properties.
The structure of matter consists primarily of three types of subatomic particles:
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutral particles, also located in the nucleus, that contribute to the mass of the atom.
- Electrons: Negatively charged particles that orbit the nucleus in defined energy levels.
These particles interact in specific ways, with the protons and neutrons forming the dense center of the atom, while the electrons orbit in various energy shells. The arrangement and number of these particles determine the identity and behavior of each element.
Key Figures in Atomic Theory
The understanding of matter’s fundamental structure has evolved through the contributions of many brilliant minds. Throughout history, scientists have developed and refined models that describe the nature of particles that make up substances. These figures have laid the groundwork for modern science by proposing revolutionary ideas that changed the way we perceive the physical world.
Among the most influential individuals, several stand out for their groundbreaking work. Their theories and discoveries have shaped the way we understand the interactions between particles and the forces that govern them.
Each figure brought new insights that, when combined, formed the foundation for current models of matter. Their work continues to impact various scientific fields, including chemistry, physics, and materials science.
Subatomic Particles Explained
The foundation of matter lies in the tiny, invisible components that make up every substance in the universe. These components, although not directly observable with the naked eye, influence the properties and behaviors of all forms of matter. Understanding these particles is essential to grasping the fundamental structure of the universe.
Electrons
Electrons are the negatively charged particles that orbit the nucleus of an atom. They are found in various energy levels around the central core and play a crucial role in chemical reactions and bonding. Despite their small mass, they are key in determining an atom’s reactivity and its interactions with other atoms.
Protons and Neutrons
Protons, which are positively charged, and neutrons, which are neutral, are located in the nucleus at the center of the atom. Together, these particles account for almost all of an atom’s mass. The number of protons in the nucleus defines the identity of the element, while neutrons help stabilize the nucleus and contribute to the atom’s overall mass.
Understanding Atomic Models
Over the centuries, scientists have developed various models to describe the structure and behavior of matter at its most fundamental level. These models have evolved as new discoveries have been made, each one refining our understanding of how particles interact and how matter is organized. While no model is perfect, each provides valuable insights into the building blocks of the universe.
The following table highlights key models and their contributors:
| Model | Scientist | Key Concept |
|---|---|---|
| Dalton’s Model | John Dalton | Atoms are indivisible and combine to form compounds in fixed ratios. |
| Thomson’s Model | J.J. Thomson | Atoms contain a positively charged “pudding” with electrons scattered inside. |
| Rutherford’s Model | Ernest Rutherford | The atom has a small, dense nucleus surrounded by a cloud of electrons. |
| Bohr’s Model | Niels Bohr | Electrons orbit the nucleus in fixed paths or energy levels. |
These models, though differing in specifics, all contributed to the current understanding of matter’s structure. As research continues, new theories and models may arise to further enhance our comprehension of the microscopic world.
Importance of the Periodic Table
The periodic table is one of the most important tools in understanding the properties and relationships of elements. It organizes all known chemical elements in a systematic way, allowing scientists to predict the behavior of substances based on their position within the table. This organization not only simplifies the study of chemistry but also provides a framework for discovering new elements and understanding complex reactions.
Key aspects of the periodic table include:
- Element Groupings: Elements are arranged into groups or families, where elements in the same column share similar properties.
- Periodic Trends: The table reveals trends in properties like electronegativity, atomic radius, and ionization energy as you move across periods and down groups.
- Predicting Reactions: By knowing an element’s position, one can often predict how it will react with other elements.
- Classification of Elements: The table divides elements into categories like metals, nonmetals, and metalloids, each with distinct characteristics.
This tool is fundamental not only for chemists but also for students and professionals in various scientific fields, enabling them to grasp the essential properties of elements and their interactions.
Electron Configuration Basics
The arrangement of electrons around an atom’s nucleus determines much of its chemical behavior and reactivity. Understanding how electrons are distributed across different energy levels or shells is essential for predicting how atoms will interact with one another. This arrangement, known as electron configuration, provides insights into the stability and reactivity of elements.
In general, electrons are organized into shells, which are further divided into sublevels. These configurations follow specific rules and patterns, such as the Pauli exclusion principle, Hund’s rule, and the Aufbau principle, which guide how electrons fill available spaces in an atom’s energy levels.
The table below shows the electron configuration for the first few elements:
| Element | Electron Configuration |
|---|---|
| Hydrogen | 1s1 |
| Helium | 1s2 |
| Lithium | 1s2 2s1 |
| Beryllium | 1s2 2s2 |
| Boron | 1s2 2s2 2p1 |
This pattern continues for all elements, with the electron configuration helping to explain the chemical properties of each element based on how its electrons are arranged. The knowledge of electron configurations is vital for understanding bonding, reactivity, and the behavior of elements in various chemical reactions.
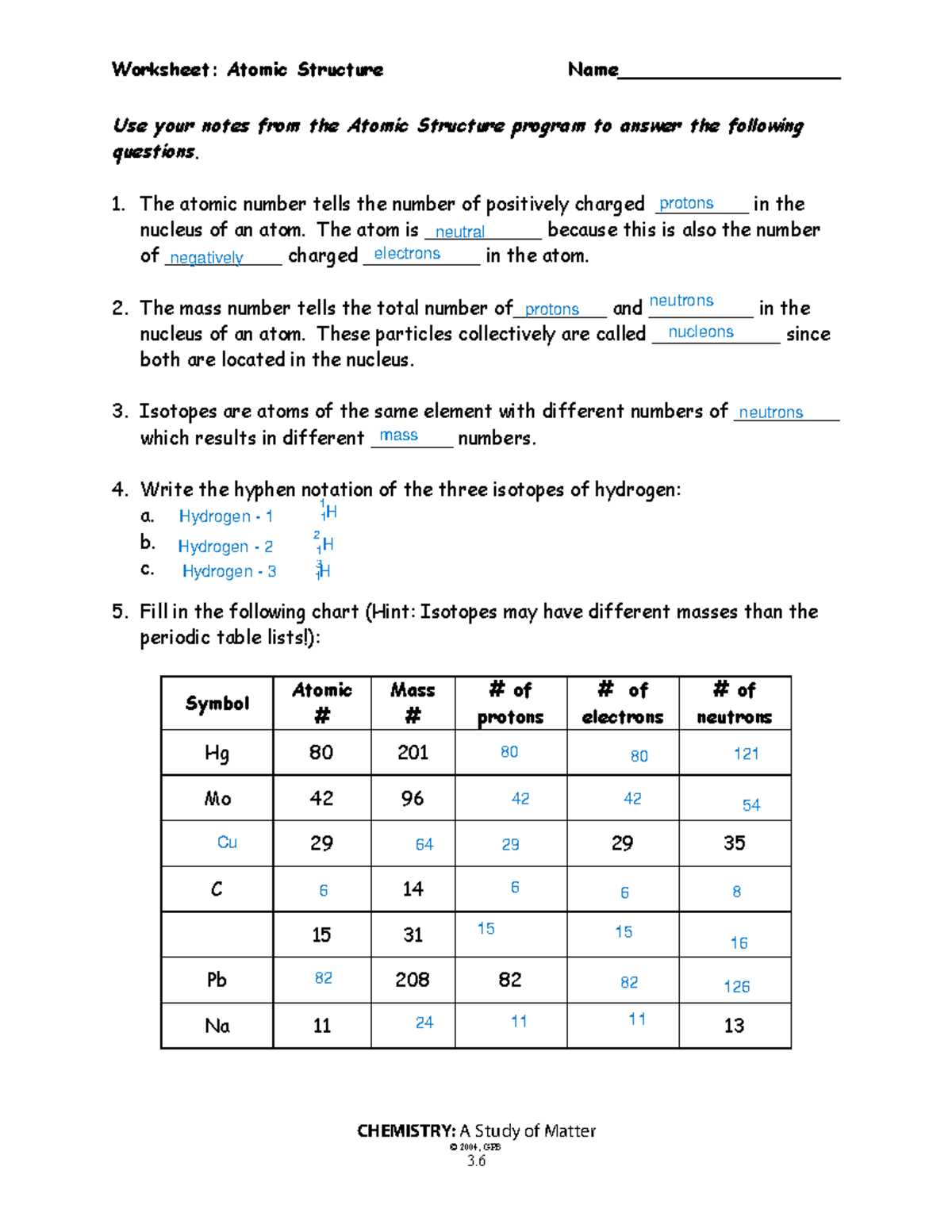
Atomic Number and Mass Number
The identity and characteristics of an element are largely determined by two key values: the number of protons in its nucleus and the total number of particles that make up its core. These two numbers play a critical role in distinguishing one element from another and in understanding the structure of matter.
There are two important properties that help define an element:
- Atomic Number: This is the number of protons in the nucleus of an atom. It uniquely identifies an element and determines its place in the periodic table.
- Mass Number: This is the sum of the protons and neutrons in an atom’s nucleus. It provides an estimate of the atom’s mass.
The atomic number is always a whole number, and it defines the element’s chemical properties. On the other hand, the mass number can vary depending on the number of neutrons present, leading to different isotopes of the same element.
For example:
- Carbon has an atomic number of 6, meaning it has 6 protons in its nucleus.
- Carbon-12 and Carbon-14 are isotopes of carbon, with mass numbers of 12 and 14, respectively, due to different numbers of neutrons.
Understanding both the atomic number and mass number is fundamental to grasping how elements behave, how they form compounds, and how they interact in chemical reactions.
Isotopes and Their Significance
Although all atoms of a given element have the same number of protons, they can differ in the number of neutrons in their nucleus. These variations lead to different forms of the same element, known as isotopes. While isotopes share many chemical properties, they can have distinct physical characteristics, such as different atomic masses and stability levels.
Natural and Artificial Isotopes
Isotopes can be classified into two categories: natural and artificial. Natural isotopes occur in nature and are often stable, while artificial isotopes are typically created in laboratories through nuclear reactions. Some natural isotopes are commonly found in elements like carbon and hydrogen, while others, such as uranium isotopes, are critical in fields like nuclear energy.
Applications of Isotopes
Isotopes have a wide range of applications in both science and industry. For example, carbon-14 is used in radiocarbon dating to determine the age of ancient objects, while iodine-131 is used in medical treatments for thyroid conditions. Additionally, isotopes are pivotal in understanding biological processes, diagnosing diseases, and generating power in nuclear reactors.
In essence, isotopes not only provide valuable insight into the properties of elements but also offer practical tools in medicine, archaeology, and energy production, highlighting their importance across various fields.
Chemical Bonds and Atomic Interactions

At the core of all chemical reactions lies the interaction between particles, which leads to the formation of various substances. Atoms, seeking to attain stability, interact by sharing or transferring electrons. These interactions result in the formation of chemical bonds, which hold atoms together in molecules or compounds. The nature of these bonds determines the properties and behaviors of the substances involved.
Types of Chemical Bonds
There are several types of bonds that form between atoms, each with distinct characteristics:
- Covalent Bonds: Formed when atoms share electrons to achieve stability. This type of bond is common in nonmetals and results in the formation of molecules.
- Ionics Bonds: Occur when one atom transfers electrons to another, creating ions that are held together by electrostatic forces. These bonds are typical in compounds formed between metals and nonmetals.
- Metallic Bonds: Found in metals, where electrons are shared collectively in a “sea” of electrons, allowing for properties like conductivity and malleability.
Importance of Atomic Interactions
The interactions between atoms are fundamental to the creation of all materials around us. From the water we drink to the air we breathe, the way atoms bond influences everything. For example, the unique properties of water, such as its high boiling point and ability to dissolve a wide range of substances, are due to hydrogen bonds between water molecules.
Understanding these interactions allows scientists to predict how different elements will combine, how reactions will proceed, and how new materials can be created for use in technology, medicine, and industry. Chemical bonds are not only essential to life itself but also drive innovation in various scientific fields.
Quantum Theory and Its Influence
In the early 20th century, a groundbreaking shift in the way we understand the behavior of matter and energy occurred. Rather than following classical laws of physics, particles at the smallest scales began to exhibit behaviors that seemed strange and unpredictable. This shift led to the development of a new framework, which has since revolutionized the fields of chemistry, physics, and technology.
At the heart of this new understanding is the concept that particles, like electrons, do not behave as tiny balls following fixed paths but instead exist in a state of probabilities. Their exact position and momentum can only be known with a certain degree of uncertainty, and they can also act as both particles and waves, depending on how they are observed. This dual nature is one of the many phenomena that emerged from this paradigm shift.
Impact on Modern Science and Technology
The influence of this new perspective is far-reaching. In the realm of chemistry, it has led to the development of sophisticated models for predicting how elements interact and bond. Quantum mechanics also explains the behavior of atoms and molecules in ways that classical theories could not, leading to a deeper understanding of the forces at play in chemical reactions.
Technological Advances Driven by Quantum Mechanics
Quantum principles have driven innovations in technology, from semiconductors and lasers to the development of quantum computers. The ability to manipulate particles at the quantum level has enabled engineers to create faster, more efficient electronic devices. Additionally, new fields like quantum cryptography are paving the way for ultra-secure communication systems, illustrating the far-reaching implications of these ideas.
In essence, the shift to a quantum view has not only reshaped our understanding of the microscopic world but has also laid the foundation for advancements that continue to transform the modern world, opening doors to new technologies that were once thought impossible.
Bohr’s Model of the Atom
In the early 20th century, the understanding of matter at the smallest scales was still incomplete. Scientists knew that atoms were the fundamental building blocks of matter, but their internal structure remained a mystery. Niels Bohr, a Danish physicist, developed a model that provided a more accurate description of atomic behavior, especially in terms of how electrons behave around the nucleus.
Bohr proposed that electrons orbit the nucleus in specific, fixed paths or “shells.” Each orbit corresponds to a particular energy level, and electrons can jump between these orbits by absorbing or emitting energy in discrete amounts. This model was a significant improvement over previous theories, as it explained the stability of atoms and the spectral lines observed in light emitted by atoms.
Energy Levels and Electron Transitions

One of the key aspects of Bohr’s model is the idea that electrons exist in quantized energy levels, or orbits, around the nucleus. Each energy level can hold a specific number of electrons, and the farther an orbit is from the nucleus, the higher its energy. When electrons move between these levels, they absorb or emit energy in the form of light. This phenomenon explains the characteristic emission spectra of different elements, where each element emits light at specific wavelengths.
Limitations and Advancements
Although Bohr’s model was groundbreaking, it was not without limitations. It worked well for hydrogen, the simplest atom, but struggled to explain the behavior of more complex elements. Later models, such as the quantum mechanical model, refined Bohr’s ideas by incorporating wave-like properties of electrons, offering a more comprehensive understanding of atomic structure.
In summary, Bohr’s model marked a pivotal moment in the development of modern atomic theory. By introducing the concept of quantized orbits and energy levels, it provided a more accurate and useful framework for understanding atomic structure, influencing both theoretical physics and experimental chemistry.
Electromagnetic Radiation and Atoms
The relationship between light and matter is central to understanding the behavior of atoms. Light, or more broadly, electromagnetic radiation, interacts with atoms in ways that reveal key information about their structure and energy states. This interaction has been extensively studied and has led to the development of various models and concepts in physics and chemistry.
Electromagnetic radiation comes in a wide range of wavelengths, from radio waves to gamma rays. Each form of radiation carries different amounts of energy, and when these waves interact with atoms, they can cause electrons to move between energy levels or states. These transitions are responsible for many observable phenomena, such as the emission spectra of different elements.
Types of Electromagnetic Radiation
Electromagnetic radiation spans a broad spectrum, with each type differing in wavelength and energy. The various types of radiation interact with atoms in distinct ways. Here is an overview of some key types:
| Type of Radiation | Wavelength Range | Energy Level |
|---|---|---|
| Radio Waves | Long wavelength (up to kilometers) | Low energy |
| Microwaves | Shorter than radio waves | Moderate energy |
| Infrared | Longer than visible light | Moderate energy |
| Visible Light | Approximately 400–700 nm | High energy |
| Ultraviolet | Shorter than visible light | High energy |
| X-rays | Very short wavelengths | Very high energy |
| Gamma Rays | Shortest wavelength | Extremely high energy |
Energy Transitions and Emission Spectra
When electromagnetic radiation interacts with atoms, it can excite electrons to higher energy levels. However, these excited states are unstable, and electrons will eventually return to lower energy levels, releasing energy in the form of light. The energy of this light corresponds to specific wavelengths, which make up the atom’s emission spectrum. Each element has a unique emission spectrum, allowing scientists to identify the composition of distant stars or analyze substances in laboratory experiments.
These transitions are critical in understanding the quantization of energy levels within an atom and the way atoms absorb and emit radiation. The precise wavelengths emitted by an atom are linked to the differences in energy between its electron shells or orbitals.
In conclusion, the study of electromagnetic radiation and its interaction with atoms has been instrumental in advancing our understanding of atomic structure. This knowledge continues to shape a wide range of scientific and technological advancements, from spectroscopy to telecommunications and beyond.
Principles of Atomic Theory in Chemistry
The foundation of chemistry lies in the understanding of the fundamental particles that compose matter. The principles governing the behavior and interaction of these particles have shaped much of modern chemistry, enabling scientists to develop models that explain the structure, reactivity, and properties of different substances. By studying these foundational concepts, we can gain a deeper insight into how atoms combine, form bonds, and participate in chemical reactions.
At the core of this understanding is the concept that matter is composed of indivisible particles, and the way these particles behave and interact under various conditions determines the chemical properties of materials. Key principles of this framework provide the basis for explaining the periodicity of elements, the nature of chemical bonds, and the outcomes of chemical reactions.
Key Principles in Chemical Reactions
The behavior of matter can be described by several fundamental ideas. These principles help explain how different substances interact and undergo transformations. Some key concepts include:
| Principle | Description |
|---|---|
| Conservation of Mass | Mass is neither created nor destroyed in a chemical reaction. The total mass of reactants equals the total mass of products. |
| Energy Conservation | Energy is conserved in a chemical process, and it can neither be created nor destroyed, only transformed between forms. |
| Electron Behavior | Electrons occupy specific energy levels or orbitals within an atom, and their transitions between these levels play a key role in chemical bonding. |
Periodic Patterns and Chemical Properties
The periodic table of elements is one of the most powerful tools for understanding the patterns that emerge when elements interact chemically. Elements are arranged in the table based on their atomic structure, with those in the same group (column) exhibiting similar chemical properties. These periodic trends are the result of the electron configurations of atoms, which determine how atoms bond and interact with each other.
In addition, the interactions between atoms are not only influenced by their individual electron configurations but also by their position within the periodic table. For instance, metals tend to lose electrons easily, while non-metals often gain electrons, leading to the formation of different types of chemical bonds.
In conclusion, the principles that govern atomic behavior form the cornerstone of chemistry. They not only explain the structure and reactivity of matter but also provide a framework for understanding the vast array of chemical phenomena that occur in nature and the laboratory.
Atomic Theory Applications in Modern Science
The understanding of the basic principles behind the structure and behavior of matter has profound implications across many areas of modern science. From medical advancements to technological innovations, the study of the building blocks of matter plays a crucial role in shaping our world. The insights derived from this field have led to the development of cutting-edge tools, techniques, and technologies that benefit numerous industries and scientific disciplines.
In contemporary science, knowledge about the fundamental particles and interactions of matter informs a range of applications, from quantum computing to advancements in materials science. By understanding how particles behave at the microscopic level, researchers can manipulate these elements to create new solutions to real-world problems.
Medical Applications
The study of matter’s fundamental components has been transformative in the field of medicine. Key applications include:
- Radiation Therapy: The use of targeted radiation in cancer treatment is based on principles related to particle interactions and energy emissions.
- Imaging Techniques: Technologies like PET scans and MRI rely on an understanding of atomic interactions to produce detailed internal images of the human body.
- Pharmacology: The design and development of pharmaceuticals often involve manipulating molecules and atoms to create compounds that can interact with the body on a molecular level.
Technological Innovations
Modern technologies, particularly those emerging in the fields of computing and materials science, benefit greatly from insights into atomic structure. Some notable applications include:
- Quantum Computing: Quantum computers leverage the principles of subatomic particles, such as electron spin, to process information at unprecedented speeds.
- Nanotechnology: Manipulating atoms and molecules at the nanoscale allows scientists to create highly precise materials with unique properties, leading to innovations in electronics, energy storage, and more.
- Semiconductors: Understanding the behavior of electrons in different materials has been key to advancing the development of semiconductor devices used in everything from smartphones to computers.
In conclusion, the study of matter at the most fundamental level continues to drive remarkable advancements in many fields. By applying the knowledge of atomic interactions, modern science has opened up new possibilities that impact both our daily lives and the future of technological development.
Review of Key Concepts and Exercises

In this section, we will review the key concepts covered in the first set of exercises, aimed at reinforcing understanding of the fundamental principles of matter. These exercises are designed to test knowledge on basic principles, definitions, and calculations related to the building blocks of matter, and how they interact with each other in various contexts.
The goal of this review is to provide clarity on some of the most important topics and address any common areas of confusion that may arise when first encountering these concepts. Below, we will summarize the main points, followed by a detailed analysis of the correct methods used to approach each question.
Key Concepts Reviewed
- Basic Definitions: Understanding the core terms such as particles, elements, and compounds.
- Particle Structure: Reviewing the properties and roles of protons, neutrons, and electrons.
- Isotopes and Elements: Exploring how different forms of the same element can have varying properties based on their atomic structure.
- Electron Configuration: Understanding how electrons are arranged in different energy levels and their effect on chemical behavior.
Common Problems and Solutions
- Misunderstanding Particle Composition: It’s common to confuse the number of protons with the number of electrons in neutral atoms. Be sure to review the periodic table for clarity.
- Confusion Around Isotopes: Remember, isotopes have the same number of protons but different numbers of neutrons, which can affect their stability.
- Electron Configuration Errors: When filling electron shells, follow the Aufbau principle, Pauli exclusion principle, and Hund’s rule to correctly assign electrons.
By revisiting these exercises and reviewing the concepts presented, learners can strengthen their understanding and application of the material. Additionally, addressing common mistakes ensures a solid foundation for more complex topics in later studies.