
Understanding the behavior of substances when exposed to high temperatures is an essential concept in the study of chemical reactions. When certain elements are heated, they emit specific colors of light that can be used to identify their presence. This technique plays a crucial role in the world of qualitative analysis, allowing scientists to distinguish between various elements based on their characteristic emissions.
Before diving into the practical aspects of this experiment, it’s important to familiarize yourself with the procedures and key concepts involved. Proper preparation is essential for accurate observations and reliable results. Knowing how to handle the necessary equipment and understanding the underlying principles will enhance your ability to identify different elements effectively.
By mastering this method, you’ll be able to recognize the unique spectral signatures of various substances, which serves as a valuable tool in both educational and professional settings. Whether you are conducting experiments in a classroom or a research facility, a clear grasp of the essential steps ensures that your experiment will be both safe and successful.
Flame Test Pre Lab Answers
In this section, we will explore the essential steps and concepts you need to grasp before conducting a high-temperature identification experiment. The goal is to understand how various substances react when exposed to heat, producing specific colors that help in recognizing different elements. Mastery of this technique requires a solid understanding of how to interpret these color changes and the factors that influence them.
Key Concepts to Review
Before performing any experiment, it is crucial to familiarize yourself with the principles behind the procedure. This includes recognizing the role of metal ions in creating unique color emissions. Each element has a distinctive reaction when subjected to heat, and knowing these patterns is vital for accurate identification. By reviewing the basic theory and expected outcomes, you will be prepared to analyze the results effectively.
Preparing for Accurate Observations
Proper preparation is essential for success. Ensure that all the necessary materials are gathered, and take time to understand how to safely handle the equipment. Even small variations in procedure can lead to discrepancies in results. Attention to detail is key to achieving clear and reliable observations during the experiment.
Understanding the Flame Test Basics

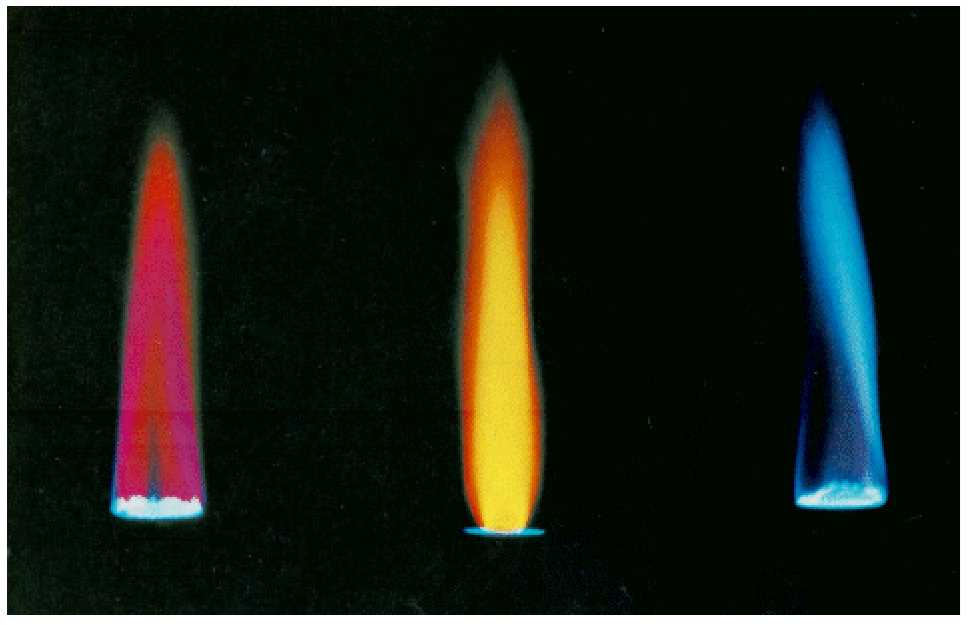
The principle behind identifying elements by heat exposure involves observing how different substances release specific colors when heated. These colors are a result of the energy emitted by electrons in the atoms of the substance as they return to their lower energy states. This phenomenon, known as atomic emission, provides a unique spectral signature for each element, which can be used for identification.
Each element produces a distinct color when exposed to high temperatures, and by carefully observing these colors, one can deduce the presence of certain metal ions in a sample. Understanding this reaction is fundamental for the experiment’s success, as it allows you to connect the colors you observe with the correct elements.
| Element | Observed Color |
|---|---|
| Lithium | Crimson Red |
| Sodium | Yellow |
| Potassium | Lavender |
| Calcium | Orange-Red |
| Barium | Green |
As seen in the table above, each metal ion emits a characteristic color when heated, which is fundamental in distinguishing between elements. Understanding these color associations is crucial for interpreting your results and ensuring accurate conclusions.
Preparing for the Flame Test Experiment
Before beginning the procedure, it is important to make sure that all necessary materials and equipment are ready and that you have a clear understanding of the process. Proper preparation is essential to ensure safety and accuracy when conducting any high-temperature reaction. This involves knowing how to handle the chemicals, how to use the equipment correctly, and understanding what to expect from the experiment’s outcomes.
Gathering Necessary Materials
To carry out the procedure effectively, you will need specific materials such as a heat source, metal salts, and a suitable observation tool. Each element you plan to test will require its corresponding substance, which will produce the desired color upon heating. Ensure that all materials are clean and free of contaminants to avoid interference with the results.
Understanding Safety Protocols
Safety is a top priority when dealing with high-temperature experiments. Always wear protective gear, such as goggles and gloves, and work in a well-ventilated area. Be mindful of the heat source and handle all materials with care to prevent accidents.
Identifying Elements by Flame Color
When various substances are heated, they emit distinct colors that can be used to identify the elements present. This occurs because of the way electrons in atoms absorb energy and then release it in the form of light as they return to lower energy states. The specific color emitted corresponds to the energy levels of the electrons in the element’s atoms, providing a unique signature for each element.
By observing the emitted colors carefully, you can determine which metal ions are present in a sample. This method is a reliable and straightforward way to analyze the composition of substances in a qualitative manner.
| Element | Color Emitted |
|---|---|
| Lithium | Crimson Red |
| Sodium | Bright Yellow |
| Potassium | Light Purple |
| Calcium | Orange-Red |
| Barium | Green |
The table above shows the typical color emissions of different metal ions when heated. Recognizing these colors during an experiment allows for the identification of elements, making this technique an essential tool in qualitative chemical analysis.
Common Metal Ions and Their Colors
When various metal ions are subjected to heat, they exhibit characteristic colors due to the release of energy as their electrons move between energy levels. These colors are unique to each element, making it possible to identify them by simply observing the light emitted. Understanding which metal ions produce which colors is essential for accurate analysis and interpretation in chemical experiments.
Key Metal Ions and Their Emissions
Different metal ions produce distinct colors when heated. For example, lithium gives off a crimson red, sodium a bright yellow, and copper a blue-green hue. These color emissions occur because each element’s electron structure is different, leading to unique wavelengths of light being emitted when the element is heated.
Applications of Metal Ion Identification
Knowing the specific color emitted by each metal ion is crucial for various scientific applications, including qualitative analysis and material testing. In practical settings, this method is widely used in laboratory experiments to identify unknown substances or to verify the presence of particular elements.
Safety Precautions During the Flame Test
Working with high temperatures and reactive substances requires careful attention to safety to prevent accidents or injuries. Ensuring proper safety measures are followed will minimize risks and ensure that the procedure is conducted smoothly. Being aware of potential hazards and how to avoid them is essential for anyone participating in this type of experiment.
Essential Safety Measures
- Always wear protective goggles and gloves to shield your eyes and hands from heat and chemicals.
- Work in a well-ventilated area to avoid inhaling fumes that may be released during heating.
- Use tongs or clamps to handle heated materials to prevent burns.
- Ensure that all flammable materials are kept away from the heat source.
- Make sure your workspace is free from clutter and distractions.
Emergency Preparedness
- Know the location of the nearest fire extinguisher and first aid kit.
- If a substance ignites unexpectedly, immediately turn off the heat source and alert the instructor.
- In case of a burn, rinse the affected area with cool water and seek medical attention if necessary.
By following these precautions, you can safely conduct the experiment and protect yourself from potential hazards.
Materials Needed for the Flame Test
To conduct a high-temperature identification experiment, several materials are essential to ensure accurate and safe results. These items are required to heat substances to the correct temperature, observe their color emissions, and maintain the integrity of the experiment throughout. Understanding the role of each material will help in preparing for a successful procedure.
Essential Equipment
Here is a list of key materials required for this experiment:
- Heat source (Bunsen burner or similar) to provide the necessary temperature for heating substances.
- Metal salts (e.g., lithium chloride, sodium chloride, potassium chloride) that will be heated to identify their unique emissions.
- Inert wire (usually platinum or nichrome) to hold the sample while it is heated.
- Protective gear (goggles, gloves) to ensure safety during the procedure.
- Clean, non-flammable surface or a heat-resistant mat to work on.
Optional Additional Materials
Depending on the complexity of the experiment, the following items may also be useful:
- A color chart to compare the observed emissions with known element colors.
- A sample holder for testing multiple substances without contamination.
Table of Common Metal Salts
| Metal Salt | Color Emission |
|---|---|
| Lithium Chloride | Crimson Red |
| Sodium Chloride | Bright Yellow |
| Potassium Chloride | Lavender |
| Calcium Chloride | Orange-Red |
| Barium Chloride | Green |
By ensuring you have all the necessary materials, you’ll be ready to safely and effectively carry out the experiment and observe the unique emissions of various elements.
How Flame Test Results Are Interpreted

The results of this experiment can be analyzed by carefully observing the color emitted when a sample is heated. The specific hue produced is linked to the energy levels of the electrons in the atoms of the substance. Different elements produce unique colors due to the variation in their atomic structure, allowing scientists to identify the elements present in a sample based on the light emitted during the reaction.
Once the sample is heated, the color observed is compared to known standards for various metal ions. This comparison allows for an accurate identification of the element or compound under investigation. A well-trained eye can distinguish between even subtle differences in color, making this a reliable method for element identification.
It is important to note that contamination, the heat source, and the method of observation can all affect the results. To ensure the most accurate interpretation, it is essential to conduct the procedure carefully, using clean materials and proper techniques.
Importance of Flame Test in Chemistry
This technique plays a significant role in chemical analysis by helping scientists identify elements based on the light emitted when they are heated. It provides a quick, simple, and effective way to examine the composition of various substances, making it a valuable tool in both educational and professional settings.
One of the key reasons this method is widely used in chemistry is its ability to provide immediate visual results. By observing the color produced when different substances are exposed to heat, researchers can rapidly determine the presence of specific metal ions without needing complex equipment or procedures.
Key Benefits of This Technique
- Quick Identification: Allows for the rapid identification of metals based on their characteristic emission spectra.
- Cost-Effective: Requires minimal equipment, making it accessible even in resource-limited environments.
- Non-Destructive: The sample remains intact after the analysis, allowing for further study if needed.
- Educational Value: Provides a hands-on approach for students to observe atomic behavior and the relationship between light and matter.
Through its simplicity and effectiveness, this method has become an essential technique in various branches of chemistry, including analytical chemistry, material science, and environmental studies.
Analyzing Flame Test Data Effectively
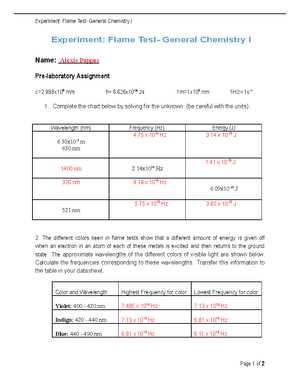
Interpreting the results of a heating experiment requires careful observation and systematic analysis. The primary goal is to match the observed color to the known emission spectra of specific elements. Accurate data interpretation relies on understanding the relationship between the observed color and the elements’ atomic structures. This ensures precise identification and minimizes errors.
Key Factors in Data Analysis
Several factors must be considered when analyzing the results of this experiment:
- Color Accuracy: Ensure that the observed color is recorded clearly and is compared to a reliable reference chart for known elements.
- Contamination: The presence of impurities can affect the color produced. Always use clean equipment to avoid misleading results.
- Heat Source: Different heat sources may influence the intensity or shade of the color. Consistency in the heating process is crucial.
Common Challenges and Solutions
Despite its simplicity, analyzing these results can present challenges, particularly when subtle differences in color are difficult to distinguish.
- Overlapping Colors: Some elements may emit similar colors. In these cases, more advanced techniques, such as using a spectrometer, may be needed for confirmation.
- Weak Emissions: If the emission is faint, increasing the temperature or adjusting the concentration of the substance may improve clarity.
By considering these factors and employing careful analysis, you can effectively interpret the results and make reliable conclusions based on the data obtained from the experiment.
Common Mistakes in Flame Test Labs
While conducting high-temperature experiments, there are several common errors that can affect the accuracy and reliability of the results. These mistakes can arise from improper handling of materials, insufficient preparation, or misinterpretation of data. Being aware of these pitfalls is essential for achieving consistent and precise outcomes.
Typical Errors During the Procedure
Some of the most frequent mistakes made during these experiments include:
- Contaminating the Sample: Using impure materials or failing to clean equipment thoroughly can result in misleading color emissions, making it difficult to identify the correct elements.
- Inconsistent Heating: Uneven heating or incorrect temperature settings can distort the color produced, leading to inaccurate observations.
- Improper Observation: Failing to observe the color change carefully or under improper lighting conditions can lead to incorrect conclusions.
Preventing Mistakes

To avoid these common errors and ensure more accurate results, consider the following tips:
- Clean Equipment: Always clean your sample holders and wires before each test to eliminate any contaminants that could affect the experiment.
- Consistent Heat Source: Use a stable and consistent heat source, adjusting the flame size as needed to ensure even heating of the sample.
- Proper Lighting: Ensure adequate lighting conditions when observing the color emissions to prevent misidentification.
By taking care to avoid these common mistakes, the reliability and precision of the experiment will improve, leading to more accurate identification of elements and better understanding of the results.
Factors Affecting Flame Test Accuracy

The precision of this experiment can be influenced by several factors that affect the reliability of the observed results. Understanding these variables is crucial to ensure accurate identification of elements. Minor discrepancies can lead to significant differences in interpretation, so it is essential to control these elements as much as possible.
Key Influencing Factors

- Contamination of Samples: Even trace amounts of contaminants on the sample can alter the emitted color, leading to erroneous conclusions.
- Heat Source Stability: Inconsistent heat intensity can result in incomplete or uneven heating, distorting the colors produced and affecting their clarity.
- Environmental Conditions: External factors, such as ambient lighting and humidity, can impact how the emitted colors are perceived and recorded.
- Equipment Quality: The condition of the materials used, such as the purity of the wires or holders, plays a crucial role in obtaining accurate results.
- Time of Observation: The length of time the sample is exposed to heat may affect the intensity and duration of the emitted color, potentially altering the final result.
Mitigating These Factors
To improve the accuracy of the results, consider the following steps:
- Use Clean Equipment: Always ensure that the sample holders and wires are thoroughly cleaned before each use to prevent contamination.
- Maintain Consistent Heating: Keep the heat source at a consistent intensity to ensure uniform exposure and avoid fluctuating emissions.
- Control Environmental Variables: Perform the experiment in a controlled environment, ensuring proper lighting and humidity levels to eliminate external interference.
By addressing these factors, the reliability of the experiment improves, leading to more accurate and reproducible results in identifying the elements present in the sample.
Using the Flame Test in Qualitative Analysis
In analytical chemistry, certain methods are employed to determine the presence of specific elements or compounds within a sample. One of these methods relies on observing the characteristic emissions produced when a sample is heated. These emissions can provide valuable insights into the chemical composition of a substance, offering a quick and reliable approach for qualitative analysis.
By carefully analyzing the emitted light and comparing it to known standards, chemists can identify the presence of various metal ions in a sample. This process is often used as an initial step in the qualitative analysis of unknown substances, particularly in the study of salts or metal-containing compounds.
Applications in Qualitative Chemistry

This method is especially useful for:
- Identifying Metal Ions: Certain metals produce distinct colors when subjected to high temperatures. This allows for quick identification of metal ions in solutions.
- Analyzing Unknown Samples: By observing the color emitted, researchers can gain initial insights into the elements present in a sample, which may then guide further analysis.
- Confirming Purity: The technique helps verify the purity of a sample by detecting impurities that might alter the expected color emissions.
Advantages and Limitations
While this method is quick and simple, there are some limitations to consider:
- Non-specificity: Some metal ions may emit similar colors, making it difficult to distinguish between them without further testing.
- Interference: Other substances in the sample can sometimes interfere with the color emissions, leading to inaccurate results.
- Limited to Metal Ions: This method is primarily useful for identifying metals and cannot be used for non-metallic substances.
Despite these limitations, the technique remains a valuable tool in the field of qualitative analysis, providing essential information that can complement other methods and help guide further investigation.
Exploring Flame Test Variations

In analytical chemistry, there are various ways to modify the basic procedure for observing the emission spectra of heated samples. These variations can enhance the precision, sensitivity, or applicability of the method, enabling it to serve a broader range of purposes. By adjusting factors like temperature, the method of introducing the sample, or the environment in which the reaction occurs, researchers can tailor the approach to suit specific analytical needs.
Temperature Control and Its Impact
One of the most common modifications is controlling the temperature at which the sample is heated. The intensity and color of the emitted light can vary depending on the heat applied, affecting the clarity of the results. Higher temperatures generally produce more vivid emissions, but excessive heat may cause the sample to decompose, leading to inaccurate or incomplete data.
Alternative Methods of Sample Introduction
Another variation involves changing how the sample is introduced to the heating source. Traditional methods use a metal loop or wire to hold the sample, but alternative techniques, such as using a platinum wire or introducing the sample directly into a Bunsen burner, can provide more consistent results. The choice of material and method can reduce contamination or allow for better handling of the sample, especially in cases where the sample may react with metal wires.
These variations allow the technique to be fine-tuned for specific applications, ensuring greater accuracy when identifying elements in different contexts. By adjusting the procedure, chemists can mitigate some of the limitations of the standard method and enhance its utility in both educational and professional settings.
Difference Between Flame Test and Other Tests
The procedure for detecting certain elements through their characteristic light emission is distinct from other methods used in analytical chemistry. While various techniques exist to identify chemical compositions, each has unique principles, advantages, and limitations. In contrast to methods like spectroscopy or chemical reactions, this particular approach relies on observing light emitted when a substance is heated, making it a relatively simple but effective qualitative method. However, its specificity and sensitivity differ significantly from other analytical techniques.
Comparing with Spectroscopy
Spectroscopy is a more precise and advanced technique for determining the elemental composition of a sample. Unlike the emission-based approach, spectroscopy involves analyzing light absorbed or emitted by atoms or molecules across a wide spectrum. This method provides more detailed information, such as the exact wavelengths of emitted light, which can be crucial for identifying trace amounts of elements. However, it requires more sophisticated equipment and higher costs compared to simpler, heat-based methods.
Comparing with Chemical Reactions
Chemical reactions for identifying elements or compounds often involve adding reagents to a sample and observing the resulting changes, such as color shifts or precipitates. While this approach can be highly specific, it may not always be as fast or straightforward as the heat-based method. Moreover, chemical tests sometimes require additional steps, such as filtration or titration, which can complicate the analysis compared to the simplicity of observing a color change during heating.
| Method | Advantages | Disadvantages |
|---|---|---|
| Heat-based Emission | Simple, fast, low cost | Less precise, only qualitative, limited to specific elements |
| Spectroscopy | Highly precise, detailed, works for trace elements | Expensive, requires specialized equipment |
| Chemical Reactions | Specific, reliable, used for a wide range of compounds | Time-consuming, may require multiple reagents and steps |
Each method offers unique strengths depending on the analysis at hand. While the heat-based procedure is valuable for quick and cost-effective identification of elements, more advanced techniques such as spectroscopy provide higher precision and are better suited for comprehensive analysis. The choice between these methods often depends on the complexity of the sample and the level of detail required in the results.
Applications of Flame Test in Real World
The method of observing light emissions when certain substances are heated plays an important role in various real-world applications. This technique provides a quick and cost-effective way to identify the presence of specific elements in a sample. From chemical analysis to educational demonstrations, its simplicity and effectiveness make it invaluable in several fields, where rapid identification and qualitative analysis are necessary. Although it is often used in laboratory settings, the versatility of this method extends to numerous practical uses in industries and research.
Use in Chemical Industries
In chemical manufacturing, this technique is frequently employed to confirm the presence of metal ions in raw materials or finished products. For instance, it can be used in quality control processes to ensure the composition of compounds, especially when elements like sodium, lithium, or copper are involved. The ability to identify metals based on their distinct light emissions helps ensure that products meet specific standards and regulations, thereby improving safety and consistency in production.
Educational Purposes and Demonstrations
This method is a cornerstone in chemistry education, where it is used to visually demonstrate the concept of atomic emission spectra. By introducing students to this hands-on technique, teachers are able to effectively explain how atoms release energy in the form of light when heated. It’s commonly featured in high school and introductory college chemistry courses, as it provides an engaging and memorable way to connect theoretical knowledge with practical experience.
Beyond the classroom, this method also finds applications in forensic analysis. For example, it can be used in the identification of metal traces in crime scene investigations, where rapid elemental analysis may be required to assess evidence. Additionally, in environmental monitoring, the technique aids in detecting trace metal contamination in water and soil samples, helping researchers and regulators maintain public health standards.
Tips for a Successful Flame Test Lab
Achieving reliable and accurate results in an experiment involving heat and the emission of light requires careful preparation and attention to detail. To ensure that the process goes smoothly, it is essential to follow a few key guidelines. Proper handling of materials, clear procedures, and a systematic approach to recording observations all contribute to a successful experiment. Whether you are working in a classroom or conducting research, these tips will help you maximize the accuracy and efficiency of your analysis.
Preparation and Setup

- Choose the Right Materials: Always use high-quality chemicals and a clean wire loop to avoid contamination. Impurities can lead to incorrect or inconsistent results.
- Calibrate Equipment: Ensure that the heat source is functioning properly and that the equipment is set up according to the protocol. An inconsistent flame can affect the accuracy of the color emissions.
- Work in a Well-Ventilated Area: Make sure the area is free from flammable substances, and work in a well-ventilated space to ensure safety throughout the procedure.
During the Experiment
- Handle Samples Carefully: Be cautious when introducing samples into the flame. Only use small amounts of material to avoid excessive smoke or unsafe reactions.
- Control Exposure Time: Exposure to the flame should be brief and controlled. Prolonged exposure can cause the element to burn off or affect the color of the emission.
- Observe and Record: Pay close attention to the color of the emitted light and record your observations immediately. This will ensure accurate data collection for later analysis.
Post-Experiment Tips
- Clean Up Thoroughly: Clean all equipment, especially the wire loop, to remove any traces of chemicals. This will prevent cross-contamination in future experiments.
- Compare Results: Cross-reference the observed colors with known data for specific metal ions. This will help verify the accuracy of your identification.
- Review Data: After the experiment, review your data and ensure consistency in your observations. If something seems off, recheck your procedure and materials.
By following these tips, you can ensure that your experiment will yield accurate and reliable results, providing valuable insights into the chemical composition of different substances.