
In this section, we delve into the core principles that shape the foundation of all living organisms. From the tiniest atoms to complex molecules, these elements are essential for sustaining life and enabling the various processes that occur within cells. Understanding these basic components is crucial for grasping how organisms grow, reproduce, and maintain balance within their environments.
Atoms form the building blocks of all matter, and their interactions give rise to molecules that are vital for countless biological functions. The intricate web of connections between these tiny particles creates the systems that keep living beings functioning efficiently. It is through the study of these interactions that we can begin to appreciate the complexity and elegance of biological processes.
By exploring the structure of essential molecules and their roles in cellular functions, we can better understand how organisms maintain stability and respond to changes. This knowledge not only aids in comprehending basic biology but also lays the groundwork for advancements in medicine, biotechnology, and environmental science.
Overview of Life’s Chemistry
This section explores the fundamental principles that govern the behavior of matter within living systems. Every organism is built upon a vast array of elements and molecules that interact in precise ways to support essential functions. From the atomic level to large macromolecules, these interactions form the backbone of all biological processes, making them crucial for maintaining health and ensuring survival.
Understanding how different substances come together to create complex structures reveals the underlying mechanisms of growth, energy transfer, and reproduction. These molecular processes allow organisms to adapt to their environments, perform vital functions, and evolve over time. The study of these foundational concepts provides valuable insight into the intricate balance that sustains all forms of life.
Understanding the Elements of Life
At the core of all biological systems are certain elements that serve as the building blocks of matter. These substances form the structure of cells and molecules that perform crucial roles in maintaining various functions within organisms. By studying these essential components, we gain insight into how complex systems emerge from basic elements to support life processes.
Key Elements in Biology
There are a few key elements that are indispensable to living organisms. Carbon, hydrogen, oxygen, and nitrogen make up most of the biological molecules in cells. These elements form bonds that allow for the creation of molecules with varying complexity, each serving unique and important purposes within organisms. Understanding their interactions helps to explain how life functions at a molecular level.
Role of Trace Elements
While major elements are vital, trace elements also play a crucial role in supporting life. These include iron, calcium, magnesium, and others that are required in small amounts. Despite their low concentration, these elements are essential for processes such as oxygen transport, muscle contraction, and enzyme activity, showcasing how even minute amounts of matter are necessary for proper function.
Atomic Structure and Its Significance
At the heart of all matter lies a fundamental unit that defines the properties and behavior of substances. This unit is composed of various smaller components, each playing a vital role in determining how atoms interact and combine to form larger structures. Understanding this basic framework provides insights into how complex molecules are formed and how they contribute to biological processes.
The arrangement of particles within an atom – protons, neutrons, and electrons – governs how it reacts with other atoms. The number of protons in the nucleus defines the element, while the arrangement of electrons influences chemical bonding and reactivity. These interactions form the basis of molecular structures that support critical functions within organisms.
Covalent Bonds and Their Role
At the heart of many essential biological processes lies a type of interaction between atoms that allows them to form stable and functional structures. This bond is crucial for creating molecules that are necessary for growth, energy production, and overall function in living organisms. These bonds are responsible for holding together the atoms that make up molecules in all forms of life.
Formation of Covalent Bonds
Covalent bonds occur when atoms share electrons to achieve a more stable electron configuration. This bond is strong, making it ideal for creating molecules that require stability for proper function. By sharing electrons, atoms can form molecules that serve specific roles within cells, tissues, and organs.
Importance in Biological Molecules
These bonds play a critical role in forming essential biological molecules. Some examples include:
- Proteins: The backbone of cellular structures and enzymes, formed by covalent bonds between amino acids.
- Carbohydrates: Energy-rich compounds created by covalent bonding between sugar molecules.
- Nucleic Acids: DNA and RNA molecules that store and transmit genetic information.
Without the stability provided by covalent bonds, these molecules would not be able to perform their functions, thus hindering biological processes.
Water’s Importance to Life Processes
Water plays a central role in sustaining all biological systems. Its unique properties allow it to support a wide range of vital processes that are essential for survival. From temperature regulation to nutrient transport, water is a critical element that enables cells to carry out the complex functions required for growth and reproduction.
One of the most significant features of water is its ability to dissolve a wide variety of substances. This makes it an ideal medium for chemical reactions that occur within cells. Water also helps in maintaining cellular structure by acting as a solvent, facilitating the movement of molecules across membranes, and contributing to metabolic processes.
Additionally, water’s high heat capacity enables organisms to regulate their internal temperature, providing stability in environments where temperature fluctuations occur. The cohesion and adhesion properties of water also allow for efficient nutrient and waste transport, ensuring that cells and tissues receive the necessary substances for energy production and removal of byproducts.
The pH Scale and Biological Reactions
Every biological process is sensitive to changes in the balance of acidity and alkalinity within an organism. The level of acidity or basicity can drastically influence how molecules interact and, consequently, how reactions proceed within cells. Understanding this balance is crucial for maintaining the stability necessary for life processes to function smoothly.
Understanding pH and Its Impact
The pH scale measures the concentration of hydrogen ions in a solution, determining whether a substance is acidic, neutral, or alkaline. In living systems, maintaining a proper pH is vital because even slight fluctuations can interfere with enzyme activity, protein structure, and other biochemical functions.
pH and Enzyme Activity
Enzymes are highly sensitive to pH changes. A change in pH can alter the shape of an enzyme, affecting its ability to catalyze reactions. The optimal pH for enzyme function varies depending on the type of enzyme, but deviations from this range can result in reduced efficiency or complete inhibition.
| Enzyme Type | Optimal pH Range |
|---|---|
| Pepsin (stomach) | 1.5 – 2 |
| Amylase (saliva) | 6.7 – 7.0 |
| Trypsin (small intestine) | 7.5 – 8.5 |
Overall, pH levels are essential for maintaining the delicate balance that allows organisms to carry out vital functions efficiently and effectively.
Macromolecules in Living Organisms
Living organisms are composed of large, complex molecules that are essential for structure, function, and regulation within cells. These macromolecules perform a wide range of tasks that are critical for maintaining cellular integrity and supporting vital biological processes. Without these large compounds, cells would not be able to carry out the functions necessary for survival.
These molecules are typically made up of repeating subunits, or monomers, which are linked together through covalent bonds to form larger, more complex structures. Each type of macromolecule has a distinct role, contributing to various cellular functions such as energy storage, information transfer, and structural support.
The four main categories of macromolecules in organisms include proteins, carbohydrates, lipids, and nucleic acids. Each type has unique characteristics and plays a specific role in cellular activities.
Proteins and Their Functions in Cells
Proteins are essential components of cells, responsible for a vast array of functions that maintain the structure and operations of living organisms. These macromolecules are made up of long chains of amino acids, and their diverse shapes enable them to perform specific tasks within the cell. From providing support to carrying out complex biochemical reactions, proteins are indispensable to cellular life.
Key Roles of Proteins in Cells
Proteins serve numerous critical functions within cells. These include:
- Structural Support: Proteins like collagen give cells and tissues their shape and rigidity.
- Catalysis: Enzymes, a class of proteins, accelerate biochemical reactions necessary for metabolism and other processes.
- Transport: Transport proteins help move molecules across cell membranes, enabling nutrient uptake and waste removal.
- Communication: Receptor proteins on cell surfaces are involved in signal transduction, allowing cells to respond to their environment.
Protein Structure and Function
The function of a protein is directly related to its three-dimensional structure, which is determined by the sequence of amino acids. Even small changes in the sequence can have profound effects on its function. This relationship between structure and function is essential for the vast diversity of tasks proteins can perform in the cell.
Carbohydrates and Their Biological Roles
Carbohydrates are essential biomolecules that serve multiple functions in living organisms. These compounds are involved in providing energy, storing fuel, and contributing to structural integrity. Their versatile nature makes them indispensable for maintaining cell function and supporting metabolic processes across different forms of life.
Energy Source and Storage
One of the primary roles of carbohydrates is to serve as a source of energy. Simple sugars, like glucose, are quickly utilized by cells for immediate energy needs. More complex carbohydrates, such as starch in plants and glycogen in animals, act as energy reserves. When needed, these stored forms can be broken down to release energy in the form of glucose, ensuring a constant supply for cellular activities.
Structural Components and Cellular Communication
Beyond energy, carbohydrates also play an important structural role. In plants, for example, cellulose strengthens cell walls, providing rigidity and support. Carbohydrates are also crucial for cell signaling, where they are often attached to proteins and lipids, forming glycoproteins and glycolipids. These molecules are involved in cell recognition, communication, and immune responses.
Lipids and Cellular Membrane Structure
Lipids are a diverse group of organic molecules that play a crucial role in the structure and function of cell membranes. These molecules are hydrophobic or amphipathic, meaning they have both water-repelling and water-attracting parts, which allows them to form the structural foundation of cellular barriers. Lipids help maintain the integrity of cells, provide protection, and facilitate communication with the surrounding environment.
Membrane Composition and Structure
The fundamental structure of cellular membranes is made up of a lipid bilayer, primarily composed of phospholipids. These molecules arrange themselves so that the hydrophobic tails face inward, shielded from water, while the hydrophilic heads are exposed to the aqueous environments on both sides. This arrangement creates a semi-permeable barrier that regulates the flow of materials in and out of the cell.
Functions of Lipids in Membranes
Lipids in cell membranes are not only structural elements but also play critical roles in signaling and energy storage:
- Signal Transduction: Lipids are involved in transmitting signals from the outside environment to the inside of the cell, helping it respond to changes.
- Energy Storage: Certain lipids, such as triglycerides, serve as long-term energy reserves within cells.
- Membrane Flexibility: Lipids contribute to the flexibility and fluidity of membranes, allowing cells to maintain their shape and function under varying conditions.
Nucleic Acids and Genetic Information
Nucleic acids are fundamental molecules that carry and store genetic information necessary for the development, functioning, and reproduction of organisms. These molecules consist of long chains of nucleotides, which encode instructions for producing proteins and other essential cellular components. They are crucial in passing genetic material from one generation to the next, ensuring continuity and diversity across all forms of life.
Structure of Nucleic Acids
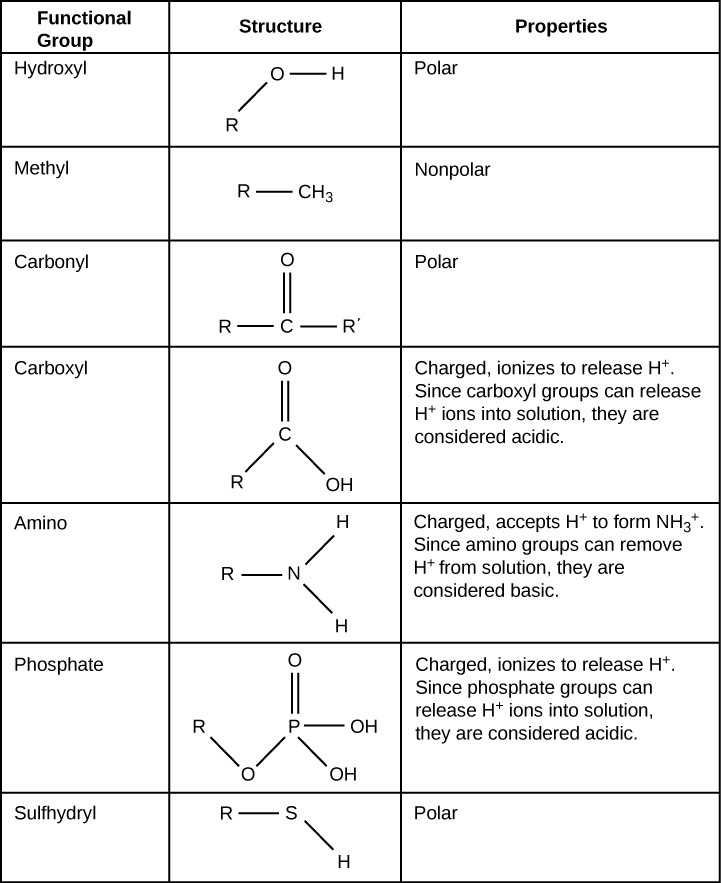
Nucleic acids come in two main forms: DNA and RNA. Both types are composed of nucleotide subunits, each containing a sugar, a phosphate group, and a nitrogenous base. In DNA, the structure is a double helix, where two strands of nucleotides coil around each other, held together by complementary base pairs. RNA, on the other hand, typically exists as a single strand and plays a key role in protein synthesis.
Role in Genetic Information Storage and Expression
Nucleic acids are essential for storing genetic blueprints and ensuring their proper expression:
- DNA: Acts as a long-term repository of genetic information, providing the instructions for cell growth, development, and function.
- RNA: Involved in translating genetic information from DNA into functional proteins that perform specific tasks within the cell.
Enzymes and Their Role in Metabolism
Enzymes are biological catalysts that accelerate chemical reactions, playing a central role in the metabolic processes that sustain life. By lowering the activation energy required for reactions to occur, enzymes enable crucial biochemical pathways to proceed at rates necessary for cell survival and function. Without enzymes, many vital reactions would occur too slowly to support the demands of the organism.
Mechanism of Action
Enzymes work by binding to specific molecules, called substrates, and converting them into products through a series of intermediate steps. This process typically occurs within the enzyme’s active site, a region specifically shaped to fit the substrate. Once the reaction is complete, the product is released, and the enzyme can catalyze further reactions without being consumed or altered.
Enzyme Regulation and Metabolic Control
The activity of enzymes is tightly regulated to ensure that metabolic processes are efficient and responsive to changes in the cell’s environment. Several factors influence enzyme function, including temperature, pH, and the presence of activators or inhibitors. Feedback mechanisms often play a critical role in controlling metabolic pathways, allowing cells to adjust their biochemical reactions based on internal and external signals.
Example: In cellular respiration, enzymes like hexokinase and pyruvate dehydrogenase catalyze key steps, controlling the flow of energy through metabolic pathways.
The Importance of Chemical Reactions
Chemical processes are fundamental to all living systems, enabling cells to carry out essential functions such as energy production, growth, and maintenance. These reactions occur constantly within organisms, driving the complex interactions between molecules that are required for survival. Without these reactions, cells would be unable to transform energy, build necessary structures, or regulate internal conditions effectively.
Key Types of Reactions in Biological Systems
In biological organisms, several types of reactions are crucial for maintaining function and health:
| Reaction Type | Description | Example |
|---|---|---|
| Synthesis Reactions | Combining smaller molecules to form larger, more complex structures. | Protein synthesis from amino acids. |
| Degradation Reactions | Breaking down larger molecules into simpler components. | Digestion of food into nutrients. |
| Exchange Reactions | Exchanging atoms or groups between molecules. | ATP hydrolysis and energy release. |
Energy Transfer in Reactions

Energy is often released or absorbed during these processes, which is vital for cellular functions. Reactions that release energy are termed exergonic, while those that require energy input are called endergonic. In cells, energy transfer is frequently mediated by molecules like ATP, which act as energy carriers, enabling the cell to power essential processes such as muscle contraction and molecular transport.
Biological Molecules and Energy Storage
Living organisms depend on a variety of molecules to capture and store energy, which is crucial for sustaining life processes. These molecules act as reservoirs, holding energy that can be accessed when needed for cellular activities such as growth, repair, and maintaining homeostasis. Understanding how these compounds function is key to unraveling the mechanisms that drive metabolism and cellular work.
Types of Energy-Storing Molecules
There are several major categories of molecules that store energy in biological systems. Each type plays a unique role in maintaining energy balance:
- Carbohydrates: Simple sugars and polysaccharides are key sources of quick energy. Monosaccharides like glucose provide an immediate energy source, while complex sugars like starch and glycogen serve as long-term energy stores in plants and animals, respectively.
- Lipids: Fats and oils store large amounts of energy in the form of triglycerides. These molecules are efficient long-term energy reserves, especially in animals, where they are stored in adipose tissue.
- Proteins: Although primarily used for structural and functional roles, proteins can also provide energy when other sources are depleted, as they can be broken down into amino acids that are processed for energy.
Energy Mobilization and Utilization
When energy is needed for cellular processes, these stored molecules are broken down through various metabolic pathways. Key mechanisms include:
- Glycolysis: The breakdown of glucose to produce ATP, the cell’s energy currency.
- Beta-oxidation: The process by which fatty acids are broken down in the mitochondria to produce acetyl-CoA, a precursor to ATP production.
- Protein catabolism: Under certain conditions, proteins can be broken down into amino acids and converted into intermediates that enter metabolic pathways.
These processes ensure that cells can continuously generate the energy needed to perform essential functions, allowing organisms to grow, respond to their environment, and sustain vital activities.
Acids, Bases, and Biological Systems
In biological systems, maintaining a balanced environment is critical for proper cellular function and overall organism health. A key aspect of this balance is the regulation of pH, which is influenced by acids and bases. These substances are fundamental to numerous biochemical reactions, affecting everything from enzyme activity to the structure of molecules. Understanding how acids and bases interact in living organisms helps explain many physiological processes, such as digestion, metabolism, and even homeostasis.
Acids and Their Role
Acids are substances that donate protons (H+) in solution, often leading to a decrease in pH. In biological contexts, acids are involved in various essential reactions. Some examples include:
- Digestive processes: Stomach acids, such as hydrochloric acid, break down food and activate enzymes necessary for nutrient absorption.
- Energy production: Acidic environments in mitochondria contribute to the production of ATP, the cell’s energy currency.
- Buffer systems: Acids help maintain a stable pH within cells and tissues by acting as part of buffering systems that prevent drastic pH changes.
Bases and Their Role

Bases, which accept protons (H+) or donate hydroxide ions (OH-) in solution, also play a crucial role in maintaining the pH balance. Their effects on biological systems include:
- Neutralization: Bases neutralize excess acids, helping to prevent damage to cellular structures and tissues.
- Enzyme regulation: Many enzymes require specific pH levels for optimal function, and bases help ensure these levels are maintained.
- Blood pH regulation: Blood contains buffer systems, including bicarbonate ions, which help maintain a slightly alkaline pH, vital for normal metabolic activities.
In summary, acids and bases are essential to maintaining the delicate balance of pH that supports life. Their roles in various biochemical processes enable organisms to carry out complex metabolic activities and maintain overall health.
Interactions Between Water and Biomolecules
Water is an essential component of living organisms, playing a central role in the structure and function of various biomolecules. The unique properties of water, such as its polarity, cohesion, and ability to form hydrogen bonds, facilitate interactions with macromolecules like proteins, nucleic acids, and lipids. These interactions are fundamental for many biological processes, including enzyme catalysis, molecular recognition, and cellular communication.
Water’s Role in Protein Structure
Proteins, as complex three-dimensional structures, rely heavily on water for their proper folding and stability. Water molecules surround and interact with hydrophilic regions of proteins, stabilizing their shape and allowing for the formation of essential functional sites. The polar nature of water also contributes to the formation of hydrogen bonds that help maintain protein structure. Additionally, water molecules are involved in:
- Hydration shells: Water molecules surround charged and polar amino acids, aiding in the proper folding of the protein.
- Enzyme function: Water helps facilitate the binding of substrates to active sites, enabling enzymatic reactions.
- Conformational changes: Water’s involvement in protein dynamics allows for the flexibility required for molecular signaling.
Water and Nucleic Acids
Water is equally vital for the structure and function of nucleic acids, such as DNA and RNA. These macromolecules rely on the solvent properties of water to maintain their helical structures and support the interactions between complementary bases. Key interactions include:
- Base pairing: Water helps stabilize the hydrogen bonds between nitrogenous bases, ensuring accurate genetic information transfer.
- Replication and transcription: Water molecules are involved in the processes of DNA replication and RNA transcription, enabling the copying and expression of genetic material.
- Solubility: Water ensures that nucleic acids remain soluble in the cell, allowing for efficient biochemical reactions.
Ultimately, water’s interactions with biomolecules are integral to life processes, maintaining the structures and functions that support cellular activity and organismal health.
The Significance of Carbon in Biology
Carbon is a fundamental element in the world of biology, serving as the backbone for a wide variety of molecules essential for life. Its unique ability to form stable, yet versatile covalent bonds with a range of other atoms allows for the creation of complex structures. This adaptability makes carbon a cornerstone of all living organisms, enabling the formation of compounds that are vital for energy storage, genetic information transfer, and cellular structure.
Carbon’s Role in Organic Molecules

Organic molecules, such as carbohydrates, proteins, lipids, and nucleic acids, all rely on carbon atoms to form their core structures. These molecules are integral to cellular function, energy production, and maintaining the integrity of biological systems. Some of carbon’s key contributions include:
- Versatility in bonding: Carbon can form single, double, and triple bonds, allowing for the creation of chains, rings, and complex branched structures.
- Formation of diverse compounds: Carbon bonds with other elements, including hydrogen, oxygen, and nitrogen, to create a wide range of molecules with varying properties and functions.
- Energy storage: Carbon atoms in carbohydrates and lipids store energy that can be released to fuel biological processes.
Carbon’s Impact on Cellular Structures
In addition to its role in forming organic molecules, carbon is integral to the structure of cellular components. For example, carbon forms the backbone of nucleic acids like DNA and RNA, ensuring the stability and replication of genetic material. Moreover, carbon is key in the formation of cell membranes, contributing to the structure and function of phospholipids, which make up the lipid bilayer.
In summary, carbon’s ability to form diverse and stable compounds is essential to biological systems, making it a vital element for the structure, function, and energy processes that sustain life.
Key Concepts for Understanding Life’s Chemistry

Understanding the fundamental principles of molecular interactions is essential for grasping the mechanisms that drive all living organisms. At the core of these processes are several important concepts that explain how molecules behave, interact, and sustain biological systems. These ideas provide a framework for studying everything from simple reactions to the complex processes occurring inside cells.
Basic Principles of Molecular Interactions
Several foundational principles help explain how biological molecules interact and contribute to life’s processes. Key ideas include:
- Energy Flow: The movement and transformation of energy between molecules, cells, and organisms is crucial for maintaining biological functions and processes.
- Intermolecular Forces: These forces, such as hydrogen bonding and van der Waals interactions, enable molecules to interact in specific ways that are necessary for cellular structure and function.
- Equilibrium and Reaction Rates: Chemical reactions must reach equilibrium to be sustainable. Understanding how rates of reaction can be influenced is vital for grasping metabolic processes.
Importance of Water and Solubility
Water is a fundamental molecule in all living systems, and its properties influence the behavior of other molecules. Key concepts related to water include:
- Polarity: Water’s polar nature allows it to dissolve a wide range of substances, making it an essential solvent for biochemical reactions.
- Hydrophilic and Hydrophobic Interactions: Molecules that are water-loving (hydrophilic) or water-fearing (hydrophobic) interact in distinct ways that affect the structure and function of cellular components, including proteins and membranes.
Macromolecular Structures and Functions
Understanding how large molecules are organized and how they perform specific tasks is critical. Key areas to focus on include:
- Proteins: These large molecules, made up of amino acids, perform a wide variety of tasks, including catalyzing reactions and providing structural support.
- DNA and RNA: These nucleic acids carry genetic information and play essential roles in protein synthesis and cell division.
- Carbohydrates and Lipids: These macromolecules provide energy, store energy, and form structural components in cells and tissues.
By understanding these core concepts, it becomes possible to explore and explain the vast complexity of biological systems and their intricate molecular machinery.