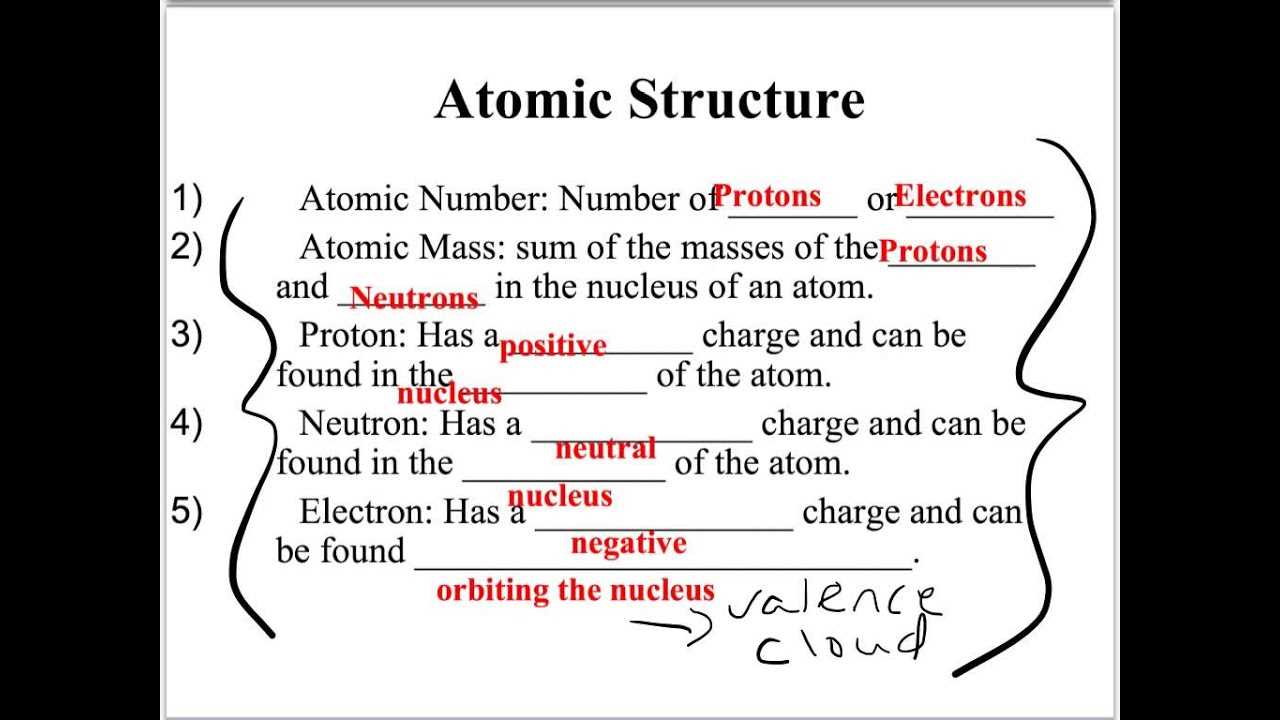
Preparing for important assessments requires a solid understanding of key principles and effective study strategies. By focusing on essential concepts and practical applications, you can build confidence and improve your performance.
In this guide, we delve into critical subject areas, offering detailed explanations and problem-solving techniques. From understanding foundational principles to tackling advanced topics, every section is designed to enhance comprehension and retention.
Whether you’re revisiting fundamental theories or exploring complex problem-solving methods, the insights provided here aim to make your learning journey more effective and rewarding. Equip yourself with the tools to excel and achieve your goals.
Key Concepts for Chemistry Exams
Understanding core principles is essential for building a strong foundation in any scientific field. By grasping the underlying ideas and their applications, you can approach problems with clarity and confidence.
One of the crucial areas to focus on is the organization and behavior of elements. Recognizing patterns and relationships between different substances can simplify complex processes. Additionally, being able to identify types of reactions and predict their outcomes is invaluable for tackling theoretical and practical questions.
Another significant aspect is the ability to perform accurate calculations. Whether it’s converting measurements or determining quantities, mastering these skills ensures precision and enhances problem-solving efficiency. Familiarity with these fundamental concepts prepares you to address a variety of challenges effectively.
Understanding Periodic Table Trends
The arrangement of elements reveals patterns that help explain their properties and interactions. Recognizing these trends makes it easier to predict behaviors and solve related problems efficiently.
One key pattern involves the way characteristics change across rows and columns. Elements in the same group often share similar traits, while shifts across periods highlight gradual changes in properties. These patterns provide a systematic approach to understanding and applying this knowledge in practical scenarios.
| Trend | Direction | Description |
|---|---|---|
| Atomic Size | Increases down a group | Atoms become larger due to added energy levels. |
| Electronegativity | Increases across a period | Elements attract electrons more strongly moving left to right. |
| Ionization Energy | Decreases down a group | Less energy is required to remove an electron as atoms grow larger. |
Familiarizing yourself with these trends allows for a deeper understanding of how elements behave and interact, enabling accurate predictions in a variety of contexts.
How Chemical Bonds Work
At the core of all substances lies the interaction between atoms, which forms the foundation of molecular structures. Understanding how atoms connect and share electrons is essential for explaining the properties and behavior of various compounds.
The most common types of atomic connections are ionic, covalent, and metallic bonds. Each type involves a different way of electron sharing or transfer, leading to distinct characteristics in the resulting substance. These interactions determine the physical and chemical properties of materials, such as their melting points, conductivity, and solubility.
For example, in an ionic bond, one atom donates electrons while another accepts them, creating charged particles called ions. This transfer results in a strong attraction between oppositely charged ions, forming stable compounds. In a covalent bond, atoms share electrons to fill their outer shells, leading to the formation of molecules. Metallic bonds, on the other hand, involve a “sea of electrons” that move freely among metal atoms, providing metals with their characteristic conductivity and malleability.
Balancing Chemical Equations Easily

Representing reactions accurately requires a clear understanding of how substances interact and transform. To ensure equations reflect the conservation of matter, each side must have the same quantity of each element.
Understanding the Basics
The process starts with identifying all the reactants and products involved. Writing their symbols and formulas sets the stage for balancing. The principle is simple: atoms are neither created nor destroyed, so the number of each type must be equal on both sides.
Step-by-Step Approach
A systematic method involves adjusting coefficients, which represent the number of molecules or units, without changing the actual formulas. Start with the most complex molecule, balance one element at a time, and leave hydrogen and oxygen for last, as they often appear in multiple compounds. Reviewing the entire equation ensures it aligns with the laws of conservation.
Practicing with different scenarios will improve speed and accuracy, making the balancing process straightforward and effective for various reaction types.
The Basics of Thermodynamics
Energy transformations play a fundamental role in understanding how systems operate and interact. These principles explain how heat and work influence physical and chemical changes, providing a framework for analyzing various processes.
Understanding Energy Flow
Thermodynamics examines the movement of energy between systems and their surroundings. Key concepts include the forms of energy involved, such as heat transfer and mechanical work. Recognizing how energy is conserved or distributed lays the groundwork for solving complex problems.
Key Principles and Laws
The foundational laws define the behavior of energy in different scenarios. The first law emphasizes conservation, stating that energy cannot be created or destroyed, only transformed. The second law introduces entropy, which measures disorder and explains why certain processes are irreversible. Together, these principles describe the natural tendencies of systems and their energetic boundaries.
Mastering these ideas allows for a deeper understanding of phenomena like phase changes, reaction spontaneity, and energy efficiency in practical applications.
Exploring Acid and Base Reactions
The interaction between certain substances can lead to significant changes in the properties of both reactants. These processes are fundamental to many chemical and biological systems, influencing everything from industrial manufacturing to metabolic functions.
Understanding pH and Reactivity
The nature of a substance’s acidity or alkalinity determines how it behaves when mixed with others. The pH scale measures this characteristic, indicating whether a substance is acidic, neutral, or basic. Acids tend to donate protons, while bases accept them, leading to different types of reactions, such as neutralization, where an acid and base combine to form water and a salt.
Neutralization and its Applications
Neutralization reactions are crucial in various applications, from everyday situations like antacid use to industrial processes. When an acid and a base react, they cancel out each other’s extremes, resulting in a more balanced substance. This principle is utilized in buffering systems that maintain stable pH levels in biological organisms and ecosystems.
By understanding these reactions, you gain insight into how substances interact under different conditions and their broader implications in both natural and engineered systems.
Steps to Solve Stoichiometry Problems
Solving stoichiometry problems involves converting between different units of measurement to understand the relationship between reactants and products in a reaction. Mastering these calculations allows for precise predictions of how much of each substance is required or produced in a chemical process.
The following steps outline a clear method to approach stoichiometric calculations:
- Write a Balanced Equation: Ensure the chemical equation is properly balanced with correct stoichiometric coefficients for each substance involved in the reaction.
- Convert Units to Moles: Use molar masses or appropriate conversion factors to express the given quantities in moles, which are essential for stoichiometric calculations.
- Use Mole Ratios: Apply the mole ratio from the balanced equation to relate the amount of one substance to the amount of another in the reaction.
- Convert Moles to Desired Units: Once the required number of moles is calculated, convert it back to the desired unit, such as grams, liters, or molecules.
By following these steps systematically, you can solve a wide range of problems with confidence and precision, whether you’re determining the amount of a reactant needed or the yield of a product.
Tips for Memorizing Polyatomic Ions
Memorizing complex ions can be a challenge, but with the right techniques, it becomes manageable. These ions are fundamental in many chemical reactions, so it’s essential to become familiar with them to work efficiently in problem-solving situations.
Here are some strategies to help retain polyatomic ions more effectively:
- Group Similar Ions: Categorize ions with similar patterns or endings, such as those ending in “-ate” or “-ite”. This approach helps you recognize them more easily in various reactions.
- Create Mnemonics: Develop mnemonic devices that link the names of ions to memorable phrases or images. For example, using a phrase that helps you remember the charge or composition of each ion can make recall easier.
- Use Flashcards: Flashcards are an excellent tool for reinforcing memory. Write the formula on one side and the name on the other, testing yourself regularly to strengthen recall.
- Practice Regularly: Consistent practice is key. The more you work with the ions, the more familiar they will become, turning them into second nature when solving problems.
By applying these techniques, you’ll be better equipped to memorize polyatomic ions and recall them when necessary for various tasks or calculations.
Significance of Chemical Reaction Types

Understanding the different categories of reactions is essential for analyzing how substances interact and transform. Each reaction type has unique characteristics that dictate the behavior of molecules, helping to predict the outcome of various processes. Recognizing these types allows for better control over reactions in both laboratory and real-world applications.
Here’s an overview of some common reaction types and their significance:
| Reaction Type | Description | Example |
|---|---|---|
| Synthesis | Two or more reactants combine to form a single product. | A + B → AB |
| Decomposition | A compound breaks down into simpler substances. | AB → A + B |
| Single Displacement | One element replaces another in a compound. | A + BC → AC + B |
| Double Displacement | Two compounds exchange components to form new compounds. | AB + CD → AD + CB |
| Combustion | A substance reacts with oxygen to produce heat and light. | CxHy + O₂ → CO₂ + H₂O |
Each of these types plays a vital role in fields ranging from industrial manufacturing to environmental processes. Understanding how and why specific reactions occur gives insight into controlling and utilizing them in practical situations.
Using Moles in Chemical Calculations
The mole concept is a fundamental tool in understanding the amount of substance involved in chemical reactions. By converting between mass, volume, and particles, it allows chemists to quantify the materials required or produced in a reaction. This concept enables the calculation of exact proportions for reactants and products, making it essential in solving practical problems in science and industry.
Steps to Use Moles in Calculations
To effectively use moles in calculations, follow these systematic steps:
- Identify Given Information: Determine what is provided in the problem, such as mass, volume, or number of particles.
- Convert to Moles: Use appropriate conversion factors, such as molar mass (for mass to moles) or molar volume (for gases at STP), to convert the given information into moles.
- Apply Mole Ratios: Utilize the mole ratio from the balanced chemical equation to relate the amount of one substance to another.
- Convert to Desired Units: After calculating the required moles, convert them back into the necessary units, such as grams, liters, or molecules.
Examples of Molar Calculations
In a typical scenario, if you are asked to find the amount of a product formed from a known amount of reactant, you would:
- Convert the given mass of reactant to moles using molar mass.
- Use the stoichiometric ratio from the balanced equation to find the moles of product.
- Convert the moles of product back into grams, if required.
By following these steps, you can accurately calculate quantities in a wide range of chemical contexts, from laboratory experiments to industrial applications.
Common Errors in Lab Experiments
During practical scientific investigations, mistakes can easily occur, leading to inaccurate results or even failed experiments. These errors can stem from various factors, including improper technique, incorrect measurements, or issues with the equipment used. Recognizing common errors can help improve the quality of the work and lead to more reliable outcomes.
Types of Common Mistakes
Here are some typical errors that can happen during laboratory work:
- Measurement Inaccuracies: Using uncalibrated or poorly functioning instruments can lead to incorrect readings. Always ensure that equipment is properly calibrated and used according to guidelines.
- Contamination: Contaminants from previous experiments or from the environment can interfere with results. It’s important to use clean glassware and other tools, and avoid cross-contamination between substances.
- Poor Timing: In reactions or processes that require precise timing, such as heating or cooling, small errors in timing can affect outcomes. Keep a close eye on the experiment and use reliable timers.
- Inconsistent Technique: Variability in technique, such as stirring speed or measurement method, can lead to variations in results. Consistent methods should be followed throughout the experiment.
- Incorrect Data Recording: Recording measurements or observations incorrectly can mislead conclusions. Always double-check data and ensure it is recorded clearly and accurately.
How to Minimize Errors
To reduce the impact of these errors, consider the following tips:
- Follow Protocols Carefully: Adhere strictly to experiment protocols and avoid shortcuts that may compromise accuracy.
- Use Proper Calibration: Calibrate equipment before use, especially if measurements are crucial to the experiment.
- Maintain Cleanliness: Ensure that all tools and surfaces are properly cleaned to avoid cross-contamination.
- Repeat Trials: Conduct multiple trials of the same experiment to check for consistency and accuracy.
By being aware of these common errors and taking steps to minimize them, you can enhance the reliability and validity of laboratory results.
How to Analyze Molecular Structures

Understanding the arrangement of atoms and bonds within a molecule is essential for predicting its properties and behavior. Analyzing molecular structures involves evaluating various aspects of the compound, from bond types to the geometry of atoms. This process allows for a deeper comprehension of how a substance interacts in different environments.
Key Aspects to Focus On
When analyzing a molecular structure, several key elements should be considered:
- Atom Connectivity: Identify how atoms are bonded to each other. This includes looking for single, double, and triple bonds between elements.
- Bond Angles: Measure the angles formed between bonds. These angles help determine the overall shape of the molecule.
- Hybridization: Understand the type of hybridization of the atoms involved, such as sp, sp², or sp³, as this influences molecular geometry.
- Polarity: Evaluate whether the molecule has a dipole moment based on its shape and the electronegativity of the atoms involved.
- Lone Pairs: Take into account any non-bonding electron pairs, as they can affect the molecule’s shape and reactivity.
Steps to Analyze the Structure
To effectively analyze a molecular structure, follow these steps:
- Draw the Lewis Structure: Begin by sketching the Lewis structure to identify all the bonds and lone pairs.
- Determine the Molecular Shape: Use VSEPR theory (Valence Shell Electron Pair Repulsion) to predict the shape based on electron pair repulsion.
- Identify Bonding and Lone Pairs: Analyze the electron pairs and bonds to assess the molecular polarity and determine its geometry.
- Check for Resonance: Verify if the molecule has resonance structures, where electron distribution can be represented in multiple ways.
By following these steps and focusing on the key aspects of the molecule’s structure, you can gain a comprehensive understanding of its properties and behavior in various chemical reactions.
Practical Applications of Chemistry Theories
Theoretical concepts in science are not just abstract ideas; they are the foundation for understanding and solving real-world problems. By applying these theories, we can improve technologies, create new materials, and enhance our daily lives in various fields. The practical use of these ideas has led to advancements in medicine, industry, and environmental sustainability.
One of the most notable applications is in medicine, where the principles of molecular interactions and reactions help in designing drugs that can target specific conditions. The study of how molecules bind and react within the body allows for the development of effective treatments for a wide range of illnesses.
In environmental science, theories related to chemical reactions are used to address pollution. By understanding how certain substances interact with the environment, scientists can develop techniques for cleaning up waste, managing emissions, and even creating renewable energy solutions. For example, the process of photosynthesis is utilized in designing more efficient solar panels.
Additionally, in the manufacturing industry, these concepts help in creating stronger materials, optimizing production processes, and reducing costs. Understanding how different compounds react under various conditions can lead to more efficient production methods and better product durability.
The application of these theories continues to shape numerous fields, demonstrating how scientific understanding can lead to tangible improvements in society. From healthcare innovations to sustainability practices, these ideas play a vital role in our modern world.
Effective Study Habits for Chemistry
Developing strong study habits is essential for mastering complex scientific concepts and performing well in assessments. Efficient learning strategies help reinforce key ideas, improve retention, and make studying more effective and manageable. By incorporating active learning techniques, time management, and a focused approach, students can gain a deeper understanding of the subject.
Key Study Strategies
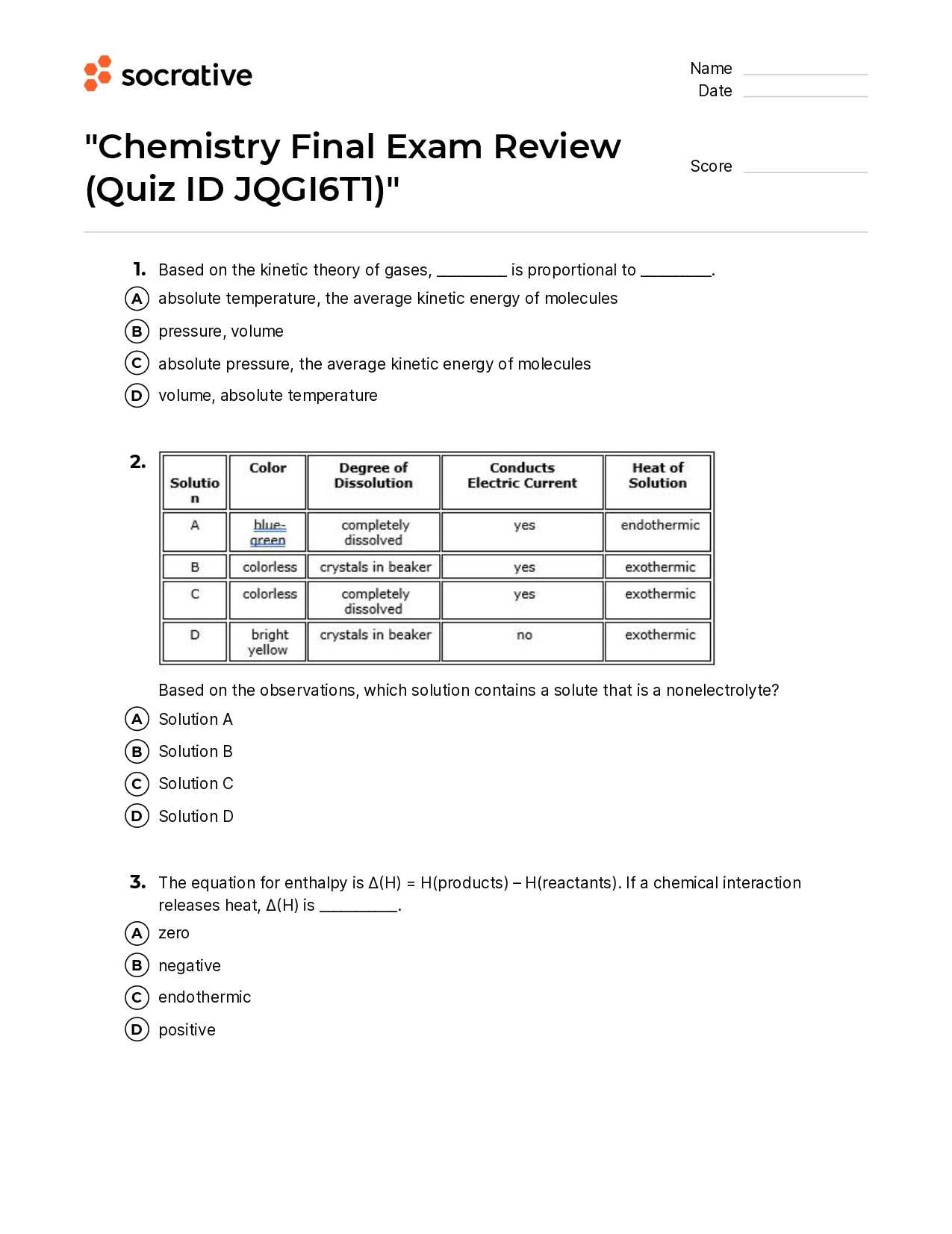
To improve retention and understanding, students can adopt the following study habits:
- Active Recall: Test yourself regularly on the material to reinforce memory. Instead of passively reading, try to explain the concepts aloud or write down key points.
- Spaced Repetition: Review the material periodically over increasing intervals to ensure long-term retention. This method is especially effective for memorizing complex formulas and terms.
- Study Groups: Collaborate with peers to discuss difficult topics. Explaining concepts to others and hearing different perspectives helps reinforce your own understanding.
- Visual Aids: Use diagrams, charts, and mind maps to visualize processes and relationships between concepts. This can make abstract ideas easier to understand.
Time Management Tips
Effective time management is key to balancing study with other responsibilities. The following tips can help you stay organized:
- Create a Study Schedule: Break down your study sessions into manageable blocks of time. Set specific goals for each session to maintain focus.
- Prioritize Difficult Topics: Tackle challenging material first when your energy and focus are at their peak. This ensures you address the hardest concepts while you’re most alert.
- Take Breaks: Regular breaks between study sessions help maintain concentration and prevent burnout. Follow the Pomodoro technique or any method that works best for you.
Using Resources Effectively
Maximize your study effectiveness by utilizing various available resources:
| Resource | How to Use |
|---|---|
| Textbooks | Use as a primary source to understand fundamental concepts. Take notes and highlight important sections. |
| Online Tutorials | Watch instructional videos and tutorials to reinforce concepts visually and hear explanations from experts. |
| Practice Problems | Solve practice problems to test your understanding and familiarize yourself with common problem-solving techniques. |
By applying these study habits, students can improve their understanding, retention, and performance, ultimately achieving success in their academic journey.
Reviewing Key Organic Chemistry Concepts
Understanding the foundational principles of organic substances is crucial for grasping how molecules interact and behave. Key topics in this area often involve various structures, reactions, and mechanisms that dictate the properties and reactivity of organic compounds. A strong grasp of these concepts is essential for both theoretical understanding and practical applications in scientific fields.
Important Concepts to Focus On
When preparing for assessments, focusing on the following fundamental topics can enhance understanding:
- Molecular Structure and Bonding: Study the different types of bonding, such as covalent and ionic, and how they influence the shape and stability of organic molecules.
- Functional Groups: Recognize the various functional groups, like alcohols, ketones, and carboxylic acids, as they play a key role in determining the chemical reactivity of organic compounds.
- Isomerism: Understand the difference between structural, geometric, and optical isomers, which have the same molecular formula but differ in structure or spatial arrangement.
- Reaction Mechanisms: Learn the step-by-step process of chemical reactions, including nucleophilic substitutions, eliminations, and additions, to predict the products of reactions.
Methods for Mastering Organic Concepts
To effectively learn and retain organic chemistry concepts, consider incorporating these study strategies:
- Practice with Models: Use molecular models or online visualizations to better understand molecular shapes and how atoms are arranged in 3D space.
- Work Through Reaction Mechanisms: Regularly practice writing out reaction mechanisms to familiarize yourself with how molecules interact during reactions.
- Use Flashcards: Create flashcards to memorize key functional groups, reaction types, and important terms, reinforcing memory retention.
- Group Study Sessions: Discuss concepts with peers to clarify difficult topics and hear different perspectives on challenging material.
By focusing on these essential concepts and using effective study techniques, students can deepen their understanding of organic substances, making it easier to analyze complex reactions and molecular structures.
Examining Environmental Chemistry Topics
Understanding the interaction between human activities and the natural world is essential for addressing environmental issues. This area explores how different substances affect the air, water, soil, and ecosystems. A key focus lies in identifying pollutants, assessing their impact, and finding sustainable solutions to mitigate environmental harm.
Important topics in this field include the study of air quality, water pollution, waste management, and the role of chemicals in climate change. By examining the properties and behavior of various compounds, it becomes possible to develop strategies for reducing harmful emissions and promoting ecological balance.
Through careful investigation of these subjects, it is possible to understand the complex relationships between industrial processes, waste production, and their effects on global health. This knowledge helps inform policy-making and environmental protection efforts worldwide.