
Preparing for an upcoming assessment in the sciences requires not only memorizing facts but also understanding underlying principles and how they apply to problem-solving. To excel, it’s essential to focus on both theory and practical applications that could appear in various question formats. A strategic approach helps you navigate through complex topics with confidence.
In this section, we will focus on enhancing your ability to tackle the most common questions you’ll face. By breaking down difficult concepts into manageable parts and refining your techniques, you’ll be better equipped to handle a wide range of challenges. With the right preparation, you can efficiently identify the key points and successfully complete tasks under time constraints.
Organizing your study sessions effectively is just as crucial as the content itself. By practicing regularly and understanding the structure of various queries, you’ll be able to improve both speed and accuracy. Emphasizing critical thinking alongside rote memorization ensures that you’re ready for any scenario.
Comprehensive Review for Success
To succeed in any science-based assessment, it’s crucial to develop a well-rounded understanding of the subject matter. This involves not only memorizing facts but also grasping the core concepts that connect the various topics. A strong foundation allows you to tackle different question formats with ease and precision, ensuring you are prepared for any challenge that may arise.
Key Topics to Focus On
Focusing on specific areas will help you feel more confident when faced with complex problems. Consider the following topics as essential building blocks:
- Understanding atomic structure and its significance in bonding
- Mastering reaction types and their balancing techniques
- Grasping thermodynamics principles and their real-world applications
- Familiarity with the periodic table and element properties
- Concentration calculations and molarity equations
Effective Study Techniques
It’s not just about what you study, but how you study. Using the following strategies will ensure you approach your preparation effectively:
- Active recall: Test yourself regularly on key concepts rather than just rereading notes.
- Practice problems: Solve problems regularly to identify common pitfalls and improve your problem-solving speed.
- Concept mapping: Create visual diagrams to connect related ideas and enhance memory retention.
- Time management: Set specific study times and avoid cramming all at once.
By following these strategies and focusing on the most relevant topics, you will build the necessary skills to approach any question with confidence and precision.
Key Topics for Success
Mastering key concepts is essential for performing well in any scientific assessment. Identifying the most critical areas of study and focusing on them strategically will help ensure that you can confidently handle the wide range of questions you may encounter. Concentrating on these core topics builds a strong foundation, enabling you to apply your knowledge effectively when solving problems.
Essential Areas of Focus
To optimize your preparation, prioritize the following topics that frequently appear in scientific assessments:
- Atomic structure and electron configuration
- Types of chemical reactions and their balancing
- Stoichiometry and mole conversions
- Acid-base theories and pH calculations
- Thermodynamic principles and energy changes
- Periodic trends and element properties
- Intermolecular forces and phase changes
Understanding Complex Concepts
Familiarity with these topics will give you an advantage, but understanding the underlying principles is equally important. Focus on:
- Problem-solving strategies: Learn how to approach different types of problems logically and systematically.
- Key equations and their applications: Make sure you can recall and apply important formulas correctly.
- Common misconceptions: Identify and address any misunderstandings that might hinder your ability to solve problems accurately.
By focusing on these core subjects and reinforcing your understanding of key principles, you’ll be well-prepared to approach any assessment with confidence and skill.
Effective Study Strategies for Success
To achieve the best results in any scientific discipline, it’s important to adopt effective study techniques that help reinforce your understanding and improve retention. The goal is not only to memorize facts but also to develop a deep comprehension of the material, allowing you to solve problems confidently and efficiently. By using the right strategies, you can streamline your preparation and maximize your chances of success.
Key Approaches to Enhance Retention
Focus on methods that encourage active engagement with the material. Consider these effective strategies:
- Active recall: Instead of rereading notes, test yourself regularly on key concepts to strengthen memory retention.
- Spaced repetition: Revisit material at increasing intervals to reinforce your knowledge over time.
- Practice problems: Solve a variety of questions to familiarize yourself with different formats and test your problem-solving skills.
- Concept mapping: Create diagrams that connect related ideas, helping you visualize relationships between concepts.
Maximizing Focus and Efficiency
In addition to mastering the content, optimizing how you study is crucial. These techniques will help you stay focused and efficient:
- Time management: Break your study sessions into focused intervals, such as 25–30 minutes of work followed by a short break.
- Prioritize weak areas: Identify topics where you struggle and devote more time to mastering them.
- Group study: Collaborate with peers to discuss challenging concepts and test each other’s knowledge.
- Minimize distractions: Create a quiet, organized study environment to improve concentration and productivity.
By incorporating these strategies into your study routine, you will not only prepare more efficiently but also build a stronger understanding of the subject, making you better equipped to tackle any challenge.
Understanding Chemical Reactions for Success
Being able to grasp the fundamentals of how substances interact and transform is essential for success in any scientific test. Chemical reactions are the core of many questions, and understanding their patterns, types, and how to balance them will help you solve problems quickly and accurately. A deep understanding of reaction mechanisms, as well as the principles that govern them, allows for easier application in various scenarios.
Types of Chemical Reactions
To tackle problems involving reactions, it’s important to recognize the different categories of reactions. Here are some common types you should be familiar with:
- Synthesis reactions: Two or more substances combine to form a more complex product.
- Decomposition reactions: A single compound breaks down into two or more simpler substances.
- Single displacement: One element replaces another in a compound.
- Double displacement: Two compounds exchange ions or elements to form new products.
- Combustion reactions: A substance reacts with oxygen, often releasing energy in the form of heat and light.
Balancing Reactions
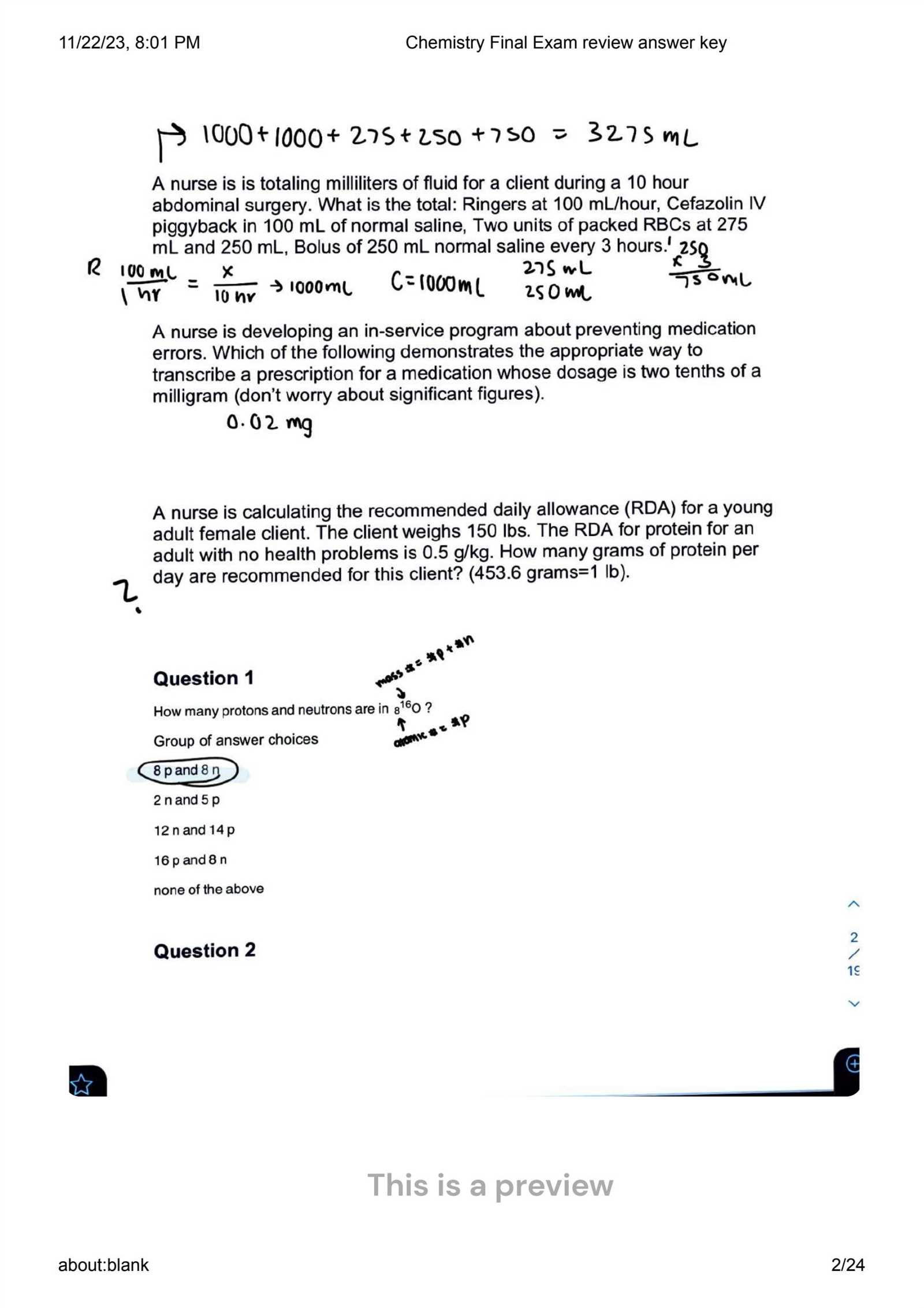
One of the most important skills in understanding reactions is balancing chemical equations. This ensures that matter is conserved and that the reaction follows the law of conservation of mass. Follow these key steps:
- Identify the reactants and products: Make sure you understand what is being combined or broken apart.
- Balance atoms: Ensure the number of atoms on each side of the equation is equal.
- Balance coefficients: Adjust the coefficients in front of compounds to achieve balance.
- Check your work: Double-check that both the mass and charge are balanced on both sides.
By mastering these concepts and practicing regularly, you will be able to approach reaction-based questions with confidence, ensuring you can quickly and accurately solve them under test conditions.
Mastering the Periodic Table Basics

Understanding the periodic table is fundamental for grasping the behavior and properties of elements. It organizes all known elements based on shared characteristics, allowing you to predict their reactions and interactions. By familiarizing yourself with its structure and the trends it exhibits, you can make connections between different groups of elements and use this knowledge to answer a wide range of questions effectively.
Element Groups and Their Properties

The table is divided into several distinct groups, each with unique traits. Knowing these groups helps in identifying patterns and predicting how elements will behave in various scenarios. Key groups to focus on include:
- Alkali metals: These elements are highly reactive and often form strong bases when combined with water.
- Halogens: Known for their reactivity, these nonmetals often form salts when combined with metals.
- Noble gases: These elements are inert and rarely form compounds due to their stable electron configuration.
- Transition metals: They are known for their ability to form a variety of compounds and play a critical role in many industrial applications.
Key Trends Across the Table
The periodic table also shows trends in properties as you move across periods or down groups. Some of the most important trends to keep in mind are:
- Atomic radius: The size of an atom decreases across a period and increases down a group.
- Electronegativity: This property increases across periods and decreases down groups, influencing how atoms attract electrons in chemical bonds.
- Ionization energy: The energy required to remove an electron from an atom increases across a period and decreases down a group.
By mastering these basic concepts and recognizing how elements relate to one another, you’ll be better prepared to understand more complex interactions and solve problems with greater ease.
Essential Concepts in Organic Chemistry
Understanding the core principles of organic compounds is crucial for solving problems related to molecular structure, reactivity, and interactions. Organic molecules are central to a wide range of scientific fields, from biology to materials science, and mastering their behavior allows you to apply this knowledge across different scenarios. Key concepts include the structure of organic molecules, functional groups, and reaction mechanisms.
Key Functional Groups
Functional groups are specific groups of atoms within molecules that determine their chemical reactivity. These groups are fundamental to understanding the behavior of organic compounds. Below is a table of some of the most common functional groups and their characteristics:
| Functional Group | Structure | Properties |
|---|---|---|
| Hydroxyl | -OH | Found in alcohols, polar, increases solubility in water |
| Aldehyde | -CHO | Highly reactive, used in oxidation reactions |
| Carboxyl | -COOH | Acidic, found in organic acids like acetic acid |
| Amines | -NH2 | Basic, can act as a nucleophile in reactions |
| Ether | -O- | Nonpolar, used as solvents in organic reactions |
Understanding Reaction Mechanisms
In organic reactions, understanding how bonds are broken and formed is essential. These reactions can be classified based on how atoms or groups are transferred between molecules. The most common mechanisms include:
- Substitution reactions: One atom or group is replaced by another.
- Elimination reactions: A small molecule is removed, usually resulting in a double bond.
- Addition reactions: Two reactants combine to form a larger molecule.
- Rearrangement reactions: The structure of the molecule is reorganized without the addition or removal of atoms.
By mastering these foundational concepts, you can better predict the behavior of organic compounds and approach related problems with confidence.
How to Approach Multiple Choice Questions

When faced with questions that offer several possible responses, it’s essential to have a strategic approach to ensure accuracy and efficiency. By understanding the structure of the questions and using effective techniques, you can significantly improve your ability to select the correct option. Knowing how to analyze each question carefully and eliminate incorrect options will give you a greater chance of success.
Effective Strategies for Answering Questions
Here are some techniques that can help you approach questions with confidence:
- Read the question carefully: Make sure you understand what is being asked before looking at the options.
- Identify keywords: Look for important terms in the question that will guide you toward the correct answer.
- Eliminate clearly wrong options: Discard any answers that are obviously incorrect to narrow down your choices.
- Consider all options: Avoid rushing to an answer without reviewing all the choices. Some options may seem correct but are misleading.
- Look for qualifiers: Words like “always,” “never,” or “usually” can give you clues about the accuracy of an option.
When You’re Unsure
If you’re unsure about a question, there are still strategies to help you make an educated guess:
- Go with your first instinct: Often, your initial answer choice is the correct one, so trust your intuition if you’re stuck.
- Use logic and reasoning: If you can eliminate at least one or two incorrect options, you increase the probability of guessing correctly.
- Review your answers: If time permits, review your responses to ensure that you’ve not overlooked any details or made careless mistakes.
By using these strategies, you can enhance your ability to efficiently navigate through questions and improve your overall performance. Confidence, careful analysis, and a methodical approach are key to answering accurately.
Top Tips for Answering Chemistry MCQs
When tackling questions with several possible options, having a clear strategy is crucial to selecting the right response. By applying effective techniques and knowing how to break down the question, you can confidently navigate through the options. Careful attention to detail and a systematic approach will greatly improve your chances of picking the correct choice.
Effective Approaches to Question Solving
Here are some key strategies that can help you successfully approach each question:
- Understand the question: Take time to fully comprehend what is being asked before moving on to the possible answers.
- Identify key terms: Focus on significant words that will help you pinpoint the correct option, such as quantities, names, or processes.
- Rule out incorrect options: Eliminate answers that are clearly wrong to narrow your choices down and increase your odds of choosing correctly.
- Don’t rush: Read each answer carefully, as some responses may be similar, and a hasty selection could lead to mistakes.
- Look for context clues: Pay attention to any hints in the question that could guide you toward the right choice.
When You’re Uncertain
If you’re faced with a question where the answer isn’t immediately clear, consider the following techniques:
- Go with your first instinct: Your initial thought is often correct. If you have a strong gut feeling, trust it and move on.
- Analyze the options: Look for common patterns or phrases in the answers to help you spot the right one.
- Review other questions: If time allows, revisit the more difficult questions after answering the easier ones. This may jog your memory and provide additional insights.
By using these tips, you can approach each question more strategically and make more confident, informed decisions during the process. Taking your time and applying these methods will enhance your performance significantly.
Common Mistakes to Avoid in Chemistry Exams
When preparing for tests involving scientific concepts, many students tend to make avoidable errors that can cost them valuable points. Understanding these common mistakes and knowing how to avoid them can greatly improve your performance. Small oversights and lack of attention to detail can often lead to incorrect conclusions, but with a bit of caution and strategy, you can minimize these missteps.
Key Mistakes to Watch Out For
- Rushing through questions: Many students rush to answer without fully reading the question, leading to misunderstanding or missing critical details. Take the time to read each question thoroughly.
- Skipping the units: Forgetting to include or convert units can result in a wrong answer, even if the calculations are correct. Always double-check your units before finalizing your response.
- Overlooking negative signs: In calculations or when dealing with formulas, negative signs are crucial. Neglecting them can change the outcome drastically, so pay close attention to positive and negative values.
- Not showing work: For problems requiring calculations, not showing your work can result in lost points, especially if the answer is incorrect. Even when unsure, outline your thought process.
- Ignoring the process of elimination: When uncertain, use the process of elimination to narrow down your options. Many students miss the opportunity to use this method effectively.
How to Overcome These Pitfalls
Avoiding common mistakes requires practice, awareness, and good test-taking strategies. Here are a few tips to help you:
- Practice under timed conditions: Simulating real test conditions can help you avoid the mistake of rushing due to time pressure.
- Review mistakes: After mock tests or practice sessions, spend time reviewing errors to ensure you don’t repeat them.
- Stay organized: Organize your thoughts and calculations before diving into the problem. Keeping a clear structure can prevent careless errors.
By staying mindful of these common mistakes, you can greatly improve your performance and approach each question with more confidence and accuracy.
How to Memorize Chemical Formulas
Memorizing chemical formulas can seem like a daunting task, but with the right strategies, it becomes much easier. Understanding the structure and patterns of compounds will help you commit them to memory more effectively. With practice and consistency, you can quickly recall formulas during assessments or when needed in practical situations.
Effective Techniques for Retention
Here are several methods you can use to help memorize chemical formulas:
- Break down the formulas: Instead of memorizing each formula as a whole, break it down into smaller components. Understand the parts that make up the formula, such as the elements and their atomic numbers, which will make it easier to recall.
- Use mnemonic devices: Mnemonics are memory aids that link information to something more familiar. Create a memorable phrase or acronym using the first letters of the elements in the formula.
- Flashcards: Create flashcards for each formula, writing the compound on one side and the formula on the other. Regularly test yourself to reinforce your memory.
- Practice regularly: Repetition is key. The more frequently you practice writing and recalling formulas, the more likely they are to stick in your memory.
Visualization and Association Techniques
Another powerful way to memorize chemical formulas is through visual techniques:
- Draw the structures: Drawing the molecular structures of compounds can help you visualize the relationship between the elements and their bonding, making it easier to recall the formula.
- Group similar formulas: Organize formulas into groups based on common patterns or similarities. This allows you to associate one formula with others that follow a similar structure, aiding memory retention.
By applying these strategies and making consistent efforts, memorizing chemical formulas will become less overwhelming and more manageable. With time and practice, you’ll develop a solid foundation that will serve you well in both theoretical and practical applications.
Preparing for Stoichiometry Problems
Stoichiometry involves calculating the quantities of reactants and products involved in chemical reactions. Understanding how to solve these types of problems is essential for success in assessments that focus on chemical calculations. By mastering the steps and developing a strong foundation in the fundamental concepts, you can tackle these problems with confidence.
Key Concepts for Stoichiometry
To approach stoichiometric calculations effectively, it is important to understand and apply the following principles:
- Balancing chemical equations: Ensure that the chemical equations are balanced before performing any calculations. A balanced equation provides the correct ratio of reactants and products needed for the stoichiometric process.
- Molarity and molar mass: Understanding how to calculate and use molar mass and molarity is crucial for converting between moles and grams, or volume and moles, depending on the type of problem.
- Conversion factors: Using conversion factors to switch between different units, such as grams, moles, and liters, is essential. Familiarize yourself with common conversion factors for gases, liquids, and solids in chemical reactions.
Strategies for Solving Stoichiometry Problems
Once you have a solid grasp of the basic concepts, follow these strategies to improve your problem-solving skills:
- Step-by-step approach: Break down each problem into manageable steps. Start with the given information, use conversion factors, and systematically work through the calculations.
- Dimensional analysis: Apply dimensional analysis to ensure that units cancel out properly and that the final result has the correct units. This method also helps avoid common mistakes.
- Practice with varied problems: The more you practice, the more familiar you will become with different types of stoichiometry problems. Work through a variety of problems to strengthen your understanding and improve speed and accuracy.
By honing your skills in stoichiometry and applying these strategies, you will be well-prepared to solve these problems with confidence and accuracy. Regular practice is key to mastering the calculations and ensuring that you can handle these challenges on assessments.
Balancing Chemical Equations Quickly

Balancing chemical equations is a crucial skill for understanding the relationships between reactants and products in a reaction. To solve these problems efficiently, it’s important to understand the key steps and use strategies that streamline the process. Mastering quick methods for balancing ensures that you can work through problems with accuracy and speed.
Steps for Efficiently Balancing Equations
Follow these steps to balance equations quickly:
- Write the unbalanced equation: Start by writing the unbalanced equation with the correct formulas for each reactant and product.
- Balance one element at a time: Focus on balancing one element at a time, starting with the most complex molecule. Often, it’s easiest to balance elements that appear in only one reactant and one product.
- Balance oxygen and hydrogen last: Oxygen and hydrogen are usually present in more than one molecule, so it’s best to balance them after the other elements are accounted for.
- Use coefficients, not subscripts: Adjust the coefficients in front of molecules to balance the equation. Do not change the subscripts in the chemical formulas, as that would alter the compounds.
- Check your work: After balancing, double-check that the number of atoms for each element is the same on both sides of the equation.
Common Strategies to Speed Up the Process
Here are some tips for speeding up the balancing process:
- Start with the most complex molecules: Begin with the molecules that contain the most elements and work your way to simpler ones. This saves time compared to balancing simpler molecules first.
- Use a systematic approach: Balance elements in the order of least to most common in the equation. Typically, balance metals first, followed by non-metals and gases.
- Practice regularly: The more you practice, the more familiar you’ll become with common patterns and shortcuts for balancing equations quickly and accurately.
By following these strategies, you can develop the skill to balance chemical equations efficiently. Regular practice and applying these methods will allow you to solve problems more quickly, leaving more time for other parts of the assessment.
Identifying Different Types of Bonds

Understanding the various types of connections between atoms is fundamental to grasping how substances interact and form new compounds. These connections, or bonds, play a critical role in determining the properties and behavior of matter. Recognizing the differences between these bonds allows for better comprehension of molecular structure and reactivity.
Types of Atomic Bonds
There are three primary types of atomic bonds that hold molecules together:
- Covalent Bonds: These bonds occur when two atoms share electrons to fill their outermost electron shells. Covalent bonds can be non-polar, where electrons are shared equally, or polar, where the sharing is unequal.
- Ionic Bonds: Ionic bonds form when one atom donates electrons to another, creating oppositely charged ions that attract each other. This type of bond is common between metals and non-metals.
- Metallic Bonds: Metallic bonds involve the sharing of free electrons among a lattice of metal atoms. This allows metals to conduct electricity and heat efficiently and gives them their characteristic malleability.
Distinguishing Between Bond Types
Here’s how to identify the type of bond in a compound:
- Electronegativity Difference: The difference in electronegativity between two atoms can determine the bond type. A large difference (usually greater than 1.7) leads to an ionic bond, while a smaller difference indicates covalent bonding.
- Element Pairing: Consider the elements involved. Non-metals typically form covalent bonds with other non-metals, while metals bond ionically with non-metals or covalently with other metals.
- Electron Movement: In covalent bonds, electrons are shared. In ionic bonds, electrons are transferred. In metallic bonds, electrons move freely between atoms.
By understanding these basic principles, you can identify the type of bond in a molecule and predict its properties, such as solubility, conductivity, and reactivity.
Key Thermodynamics Principles for the Exam
Understanding the fundamental concepts of energy transformations is crucial when studying systems and their interactions. These principles provide insight into how energy flows within physical processes and the factors that influence such transformations. Mastering these key ideas will help in solving problems related to heat, work, and energy transfer.
Essential Thermodynamics Laws
There are four major laws in thermodynamics that govern the behavior of energy in various systems:
- First Law: Also known as the Law of Energy Conservation, this principle states that energy cannot be created or destroyed, only transformed from one form to another. The total energy of an isolated system remains constant.
- Second Law: This law introduces the concept of entropy, which measures the disorder of a system. It states that the entropy of an isolated system always increases over time, meaning that natural processes tend to move towards greater disorder.
- Third Law: This law suggests that as the temperature of a system approaches absolute zero, the entropy of the system approaches a minimum value. It essentially establishes a reference point for measuring entropy.
- Zeroth Law: The Zeroth Law of Thermodynamics establishes the concept of temperature. It states that if two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
Important Concepts to Understand
Alongside the laws, there are key terms and concepts that are essential to solving problems in thermodynamics:
- Enthalpy (H): This refers to the total heat content of a system. It is useful in understanding heat flow during chemical reactions, especially at constant pressure.
- Gibbs Free Energy (G): This function helps predict whether a process will occur spontaneously. A negative change in Gibbs Free Energy indicates a spontaneous process.
- Work and Heat: These are the two primary ways that energy is transferred into or out of a system. Work is done when a force is applied over a distance, while heat is the transfer of energy due to temperature difference.
- Internal Energy (U): This is the total energy contained within a system, including kinetic and potential energies of molecules and atoms.
By thoroughly understanding these laws and concepts, you will be able to approach thermodynamics problems with confidence and precision, providing clarity in solving energy-related questions.
Understanding Acids and Bases Concepts
The behavior of certain substances when dissolved in water plays a critical role in a wide range of chemical reactions. These substances can either donate or accept protons, altering the pH of the solution. Understanding the basic principles behind these reactions is essential for solving many types of questions, especially when working with neutralization, titration, and buffering systems.
Key Definitions and Concepts
Acids and bases are defined in several ways, and knowing the different models will help in identifying the properties and behavior of substances in various scenarios. Below are some fundamental ideas:
| Concept | Description |
|---|---|
| Arrhenius Acid | A substance that increases the concentration of hydrogen ions (H+) in an aqueous solution. |
| Arrhenius Base | A substance that increases the concentration of hydroxide ions (OH-) in an aqueous solution. |
| Bronsted-Lowry Acid | A substance that donates a proton (H+) to another substance. |
| Bronsted-Lowry Base | A substance that accepts a proton (H+) from another substance. |
| Lewis Acid | A substance that accepts an electron pair. |
| Lewis Base | A substance that donates an electron pair. |
pH and pOH Concepts
The strength of an acid or base can be quantified by measuring its pH or pOH. These values are directly related to the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in a solution. Understanding how to calculate these values and interpret them is key to answering related questions.
- pH: A measure of the hydrogen ion concentration in a solution. A low pH indicates an acidic solution, while a high pH indicates a basic solution.
- pOH: A measure of the hydroxide ion concentration. It is related to pH by the equation: pH + pOH = 14.
By familiarizing yourself with these definitions and calculations, you’ll be better prepared to identify acids and bases in a variety of reactions, making it easier to approach problems involving neutralization, equilibrium, and buffer solutions.
Time Management Tips for Chemistry Exams
Effective time management is a crucial skill when preparing for any test, especially when the material is complex and requires a deep understanding of different concepts. Having a clear strategy for allocating time during your preparation and on the day of the assessment can significantly reduce stress and improve performance. By managing time wisely, you can ensure that every topic receives the attention it needs while leaving enough time for review and practice.
Pre-Test Preparation

When it comes to preparing for the assessment, time management should start well before the actual test day. A strategic plan will allow you to cover all the necessary material without cramming. Consider the following tips:
- Create a Study Schedule: Break down the study material into manageable sections. Allocate specific time slots for each topic based on its complexity and importance.
- Prioritize Key Topics: Focus on areas that are most likely to appear or that you struggle with the most. Make sure to review both your strengths and weaknesses.
- Practice Regularly: Dedicate time to solving practice problems and mock assessments. The more you practice, the more efficient you will become in answering questions under time constraints.
Test-Day Strategy
On the day of the assessment, managing your time effectively can make a big difference in your ability to complete the test with confidence. Follow these strategies to maximize your performance:
- Read Instructions Carefully: Begin by thoroughly reading all instructions and questions. This will prevent misunderstandings that can waste precious time.
- Time Allocation: Decide how much time you should spend on each section or question. Allocate more time to complex questions and less to simpler ones.
- Don’t Get Stuck: If you’re stuck on a question, move on and come back to it later if time permits. This ensures you don’t waste time on questions that could potentially lower your overall score.
By following these strategies, you’ll be able to tackle your upcoming assessment with confidence and efficiency. Mastering the art of time management is key to handling both the preparation and the test itself effectively.