When studying the behavior of liquids under various conditions, one key phenomenon involves the transformation from liquid to gas. This process is influenced by a variety of factors that govern how particles interact with one another, ultimately affecting the rate and efficiency of the transition. By exploring these interactions, we can gain deeper insight into the fundamental principles that govern matter in different states.
Through controlled experiments, it becomes possible to observe how different variables, such as temperature or surface area, can alter the rate of change from liquid to vapor. Understanding these effects is crucial for comprehending not only basic scientific concepts but also their practical applications in everyday life and industry. These investigations reveal the importance of molecular forces in shaping the behavior of substances as they undergo phase changes.
In the following sections, we will explore the methodology used to study these phenomena, provide an overview of the factors at play, and discuss how the results from such experiments contribute to our broader understanding of material properties. By examining data and applying theoretical knowledge, it becomes clear how minute interactions between particles can influence larger-scale outcomes.
Evaporation and Intermolecular Attractions Lab Report Answers
This section focuses on the key findings and insights gained from the experiment examining how particles in liquids interact with each other under various conditions. By studying how these forces affect the transition from liquid to gas, we can gain a deeper understanding of molecular behavior and how different variables impact the rate of change.
Key Findings and Observations
The experiment revealed several important aspects about the transformation process. Key points include:
- The effect of temperature on the rate of phase transition.
- The role of surface area in facilitating the change from liquid to vapor.
- How particle size and type influence the efficiency of the transition.
Factors Influencing Molecular Behavior
In the experiment, several factors played a significant role in determining the speed and extent of the transition. These include:
- Temperature: Higher temperatures provide more energy for particles to break free from the liquid state.
- Surface Area: A larger surface area allows for a greater number of particles to be exposed to the environment, speeding up the process
The Science Behind Intermolecular Forces
The behavior of substances, particularly in the transition between different states of matter, is largely influenced by the forces acting between molecules. These forces govern how particles interact, hold together, or break free from each other when exposed to external conditions. Understanding these interactions provides insight into a wide range of physical phenomena, from boiling points to solubility.
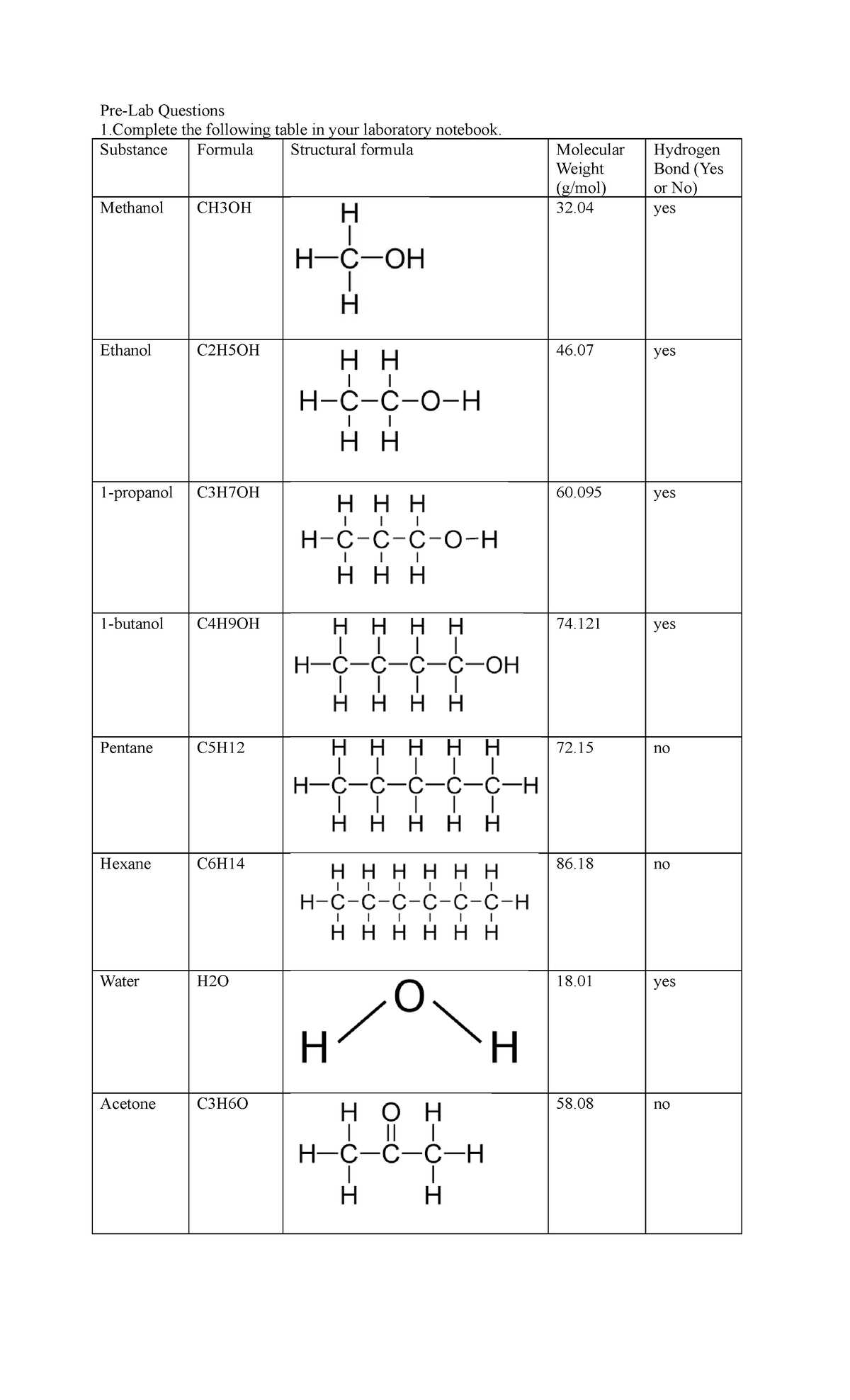
Types of Molecular Interactions
There are several types of forces that govern how molecules interact. Each type varies in strength and affects the properties of substances differently. These interactions include:
Force Type Description Effect on Substances Van der Waals Forces Weak interactions arising from temporary dipoles in molecules. Influence properties like boiling point and surface tension. Dipole-Dipole Interactions Forces between polar molecules with permanent dipoles. Strengthen interactions between polar substances, affecting their behavior in solutions. Hydrogen Bonding A special type of dipole interaction involving hydrogen and electronegative atoms like oxygen or nitrogen. Crucial in determining the boiling points of water and other liquids with hydrogen bonds. How These Forces Influence Physical Properties
The strength and nature of molecular forces directly impact several physical characteristics of substances. These include:
- Melting and Boiling Points: Stronger forces require more energy to overcome, resulting in higher melting and boiling points.
- Viscosity: The more substantial the molecular attraction, the thicker the substance, as molecules resist flowing past each other.
- Surface Tension: Molecules that are strongly attracted to each other increase surface tension, making it harder for the liquid to spread.
Factors Affecting Evaporation Rates
The rate at which a liquid changes into a gas is determined by several factors that influence how molecules at the surface escape into the surrounding environment. Understanding these variables is important for both scientific investigations and real-world applications, such as in cooling systems or natural processes like water cycles.
Key Factors Influencing the Rate
Several conditions directly affect how quickly a liquid transitions to vapor. These include temperature, surface area, air movement, and the characteristics of the liquid itself.
Factor Effect on Rate Explanation Temperature Increases rate Higher temperatures give molecules more energy, making it easier for them to escape from the liquid phase. Surface Area Increases rate A larger surface area exposes more molecules to the air, allowing for faster escape into the atmosphere. Air Movement Increases rate Moving air helps remove vapor molecules from the liquid surface, allowing more molecules to transition into the gas phase. Liquid Characteristics Varies Liquids with weaker molecular bonds tend to transition more easily, while liquids with stronger bonds require more energy. Additional Variables to Consider
Other factors, such as atmospheric pressure and humidity, also play a role in the rate of change. For instance, increased humidity slows down the process because the air already contains more vapor, reducing the ability of the liquid to release molecules. Similarly, changes in atmospheric pressure can influence the energy needed for molecules to leave the liquid state.
Understanding Molecular Interaction and States
The behavior of substances in various conditions is largely governed by how their molecules interact. These interactions determine whether a material is solid, liquid, or gas, and how it responds to external forces such as heat or pressure. Understanding the fundamental forces at play allows scientists to predict how materials will behave and transition between states under different circumstances.
The Dynamics of Molecular Forces
The strength and nature of the forces between molecules are crucial in determining the state of a substance. In solids, molecules are held closely together by strong forces, leading to a rigid structure. In liquids, these forces are weaker, allowing molecules to flow and take the shape of their container. In gases, the forces are minimal, allowing molecules to move freely and spread out.
Phase Changes and Molecular Behavior
Changes in temperature or pressure can cause substances to transition from one state to another. For example, heating a liquid increases the energy of the molecules, making it easier for them to break free and enter the gas phase. Similarly, lowering the temperature slows molecular movement, causing substances to solidify as the particles move closer together. Understanding these processes is vital in fields ranging from chemistry to engineering.
Key Observations in Evaporation Experiments
In experiments focused on the transition of a liquid to a gas, several critical factors should be monitored to understand the underlying processes. These observations reveal how different conditions influence the rate at which molecules leave the liquid phase and enter the surrounding environment. Identifying these factors helps in refining experimental setups and interpreting results accurately.
Important Factors to Observe
- Temperature: A noticeable change in temperature often correlates with an increase in the rate of transition. Higher temperatures provide more energy to the molecules, encouraging them to move faster and escape into the air.
- Surface Area: The size of the exposed surface directly impacts the speed of the process. A larger surface area allows more molecules to interact with the surrounding air, speeding up the transition.
- Air Movement: Moving air removes vapor molecules from the liquid’s surface, making it easier for additional molecules to escape. This results in a faster process when airflow is increased.
- Time: Over time, as molecules leave the liquid, the remaining substance may change in temperature or volume, which can be used to assess the rate of transition.
Common Results and Patterns
- Gradual Decrease in Liquid Volume: As more molecules leave the liquid phase, the volume of the substance decreases, which can be measured throughout the experiment.
- Formation of Condensation: In some setups, vapor molecules may condense on nearby surfaces, offering visual evidence of the process at work.
- Rate Consistency: Under controlled conditions, the rate of transition often becomes stable after a certain period, reflecting equilibrium between the liquid and gas phases.
Types of Intermolecular Forces in Detail
The forces that hold molecules together play a significant role in determining the physical properties of substances, such as boiling and melting points, viscosity, and solubility. These forces arise from the interactions between different molecules and can vary in strength and nature. Understanding these forces is crucial for explaining how substances behave in various environments.
Common Types of Molecular Interactions
- Dipole-Dipole Forces: These forces occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another. They are generally stronger than London dispersion forces but weaker than hydrogen bonds.
- Hydrogen Bonds: A specific and stronger type of dipole-dipole interaction, hydrogen bonds form when a hydrogen atom, bonded to a highly electronegative atom like nitrogen, oxygen, or fluorine, is
The Role of Energy in Evaporation
Energy plays a crucial role in the transition of a substance from its liquid state to a gas. The process requires molecules in the liquid phase to overcome the forces binding them together and escape into the air. This energy is usually supplied in the form of heat, which increases the movement of molecules and allows them to break free from the liquid’s surface. Understanding how energy affects this process is key to explaining various physical phenomena.
How Heat Affects Molecular Movement
When heat is applied to a liquid, the molecules within gain energy. This increased energy causes the molecules to move faster, reducing the attractive forces between them. As the molecules gain enough kinetic energy, some will have enough force to break away from the liquid and enter the gas phase. The higher the temperature, the faster the molecules move, which increases the likelihood of this transition occurring.
Energy Transfer and Phase Change
During this transition, energy is absorbed by the liquid without a rise in temperature until the phase change is complete. This energy is used to overcome the potential energy barrier that keeps the molecules bound together. The process continues until equilibrium is reached or until the substance completely changes phase, depending on the conditions in place.
Evaporation and Its Temperature Dependence
The rate at which a liquid transforms into a gas is significantly influenced by temperature. As the temperature of the liquid increases, the kinetic energy of the molecules also rises, causing them to move more rapidly. This increased molecular motion makes it easier for molecules to break free from the liquid’s surface, thereby accelerating the process of turning into vapor. Understanding the relationship between temperature and this transition is essential in many scientific fields and practical applications.
Effect of Temperature on Molecule Movement
At higher temperatures, molecules within a liquid possess greater energy, which allows them to overcome the forces binding them to other molecules. The increased speed of molecules causes more frequent and energetic collisions, increasing the chances that a molecule will escape the surface. Conversely, at lower temperatures, the molecules move more slowly, and fewer molecules have enough energy to escape the liquid phase.
Quantifying the Influence of Temperature
One way to understand the effect of temperature on the rate of change from liquid to gas is through the concept of vapor pressure. As temperature rises, the vapor pressure of a liquid also increases because more molecules are able to escape into the air. Below is a table summarizing the general trend of vapor pressure with temperature changes for a common substance.
Temperature (°C) Vapor Pressure (mmHg) 20 2.3 40 7.4 60 19.9 80 47.4 This table demonstrates how the vapor pressure increases with rising temperature, indicating a greater rate of molecular transition into the vapor phase. Therefore, temperature is a critical factor in determining how quickly and effectively a liquid can undergo this transformation.
How Surface Area Influences Evaporation
The process by which a liquid transitions to a gaseous state is affected by the area exposed to the surrounding environment. A larger surface area provides more opportunity for molecules to escape from the liquid phase. This concept is essential when considering how quickly a substance can undergo phase change. The greater the exposed surface, the more molecules are able to break free, speeding up the process.
The Relationship Between Surface Exposure and Molecule Release
When a liquid is spread over a larger area, there are more molecules at the surface, which are the first to escape into the gas phase. The increased number of surface molecules allows for a higher rate of transition as they absorb enough energy to break free from the liquid. In contrast, a smaller exposed surface area means fewer molecules are available to make this transition, resulting in a slower process.
Practical Examples of Surface Area Effects
Consider the difference between a puddle and a thin film of liquid. A puddle has a much smaller exposed surface compared to the same volume spread out as a thin layer, meaning it will take longer for all the liquid to transition into vapor. Similarly, when liquids are stirred or agitated, surface area increases, leading to a faster rate of molecular escape.
The Relationship Between Evaporation and Boiling
Both processes involve the transition of a liquid into a gas, but they occur under different conditions and involve distinct mechanisms. While both phenomena rely on the movement of molecules from the liquid phase to the gas phase, the primary difference lies in how and where this transition takes place. Understanding the connection between these two processes can provide insights into the behavior of substances under various temperature and pressure conditions.
Key Differences in the Processes
The primary distinctions between these two processes can be summarized as follows:
- Evaporation: Occurs at the surface of a liquid at any temperature. Molecules with sufficient energy at the surface can escape into the surrounding atmosphere.
- Boiling: Occurs throughout the entire liquid when it reaches its boiling point. At this temperature, the vapor pressure equals the external pressure, and bubbles of vapor form within the liquid.
How Temperature Affects Both Processes

Temperature plays a critical role in both phenomena. As the temperature of a liquid increases, more molecules acquire the necessary energy to escape the liquid phase. In the case of boiling, this leads to the formation of bubbles throughout the liquid, whereas in evaporation, molecules at the surface gain enough energy to transition into the gas phase. However, boiling only occurs at a specific temperature (the boiling point), whereas evaporation can happen at a range of temperatures.
Measuring Evaporation Rate in Labs
In experimental settings, determining how quickly a liquid transforms into a gas is essential for understanding its behavior under varying conditions. This measurement is crucial for applications in both scientific research and industrial processes. Several methods can be employed to assess the rate at which molecules leave the liquid phase, depending on the factors being studied and the level of precision required.
Typically, this process involves monitoring the change in mass or volume of a liquid over time. The faster the liquid loses mass or volume, the higher the rate of transition to the gaseous state. To accurately assess this, factors such as temperature, air movement, and surface area must be carefully controlled and measured.
Common approaches include using a balance to measure mass loss or setting up controlled environments where temperature and airflow are manipulated to observe their influence on the rate of change. These methods can help quantify the process and provide valuable data for further analysis.
Measuring Evaporation Rate in Labs
In experimental settings, determining how quickly a liquid transforms into a gas is essential for understanding its behavior under varying conditions. This measurement is crucial for applications in both scientific research and industrial processes. Several methods can be employed to assess the rate at which molecules leave the liquid phase, depending on the factors being studied and the level of precision required.
Typically, this process involves monitoring the change in mass or volume of a liquid over time. The faster the liquid loses mass or volume, the higher the rate of transition to the gaseous state. To accurately assess this, factors such as temperature, air movement, and surface area must be carefully controlled and measured.
Common approaches include using a balance to measure mass loss or setting up controlled environments where temperature and airflow are manipulated to observe their influence on the rate of change. These methods can help quantify the process and provide valuable data for further analysis.
Common Mistakes in Evaporation Experiments
When conducting experiments that involve the transition of substances from liquid to gas, there are several common errors that can impact the accuracy of results. These mistakes often arise due to improper handling, measurement inconsistencies, or failure to account for environmental variables. Understanding these pitfalls is crucial for achieving reliable and reproducible results in such studies.
One of the most frequent mistakes is failing to maintain a controlled environment. Factors like temperature, humidity, and air circulation can significantly influence the process and may lead to skewed data. Another common issue is the inaccurate measurement of time or mass, which can distort the observed trends and affect the conclusions drawn from the experiment.
Typical Errors to Avoid
- Not controlling temperature or humidity levels
- Inconsistent measurement of the liquid amount or time intervals
- Failure to use proper calibration for measuring instruments
- Overlooking external factors such as air movement or light exposure
- Incorrect recording of data leading to inaccurate analysis
Impact of Inaccuracies on Results

Inaccuracies in measurement or environmental control can lead to misleading results, affecting the interpretation of how quickly substances transition into the gaseous state. For instance, if the temperature fluctuates during the experiment, it may cause variations in the rate of change, resulting in data that does not reflect the true behavior of the system being studied.
By being mindful of these common errors, researchers can enhance the reliability of their experiments and ensure that their conclusions are based on accurate and consistent data.
The Effect of Molecular Size on Evaporation
The size of molecules in a liquid can significantly impact the speed at which they transition into the gas phase. Generally, the larger the molecules, the stronger the forces between them, making it harder for them to break free from the liquid. This means that liquids composed of larger molecules typically take longer to transition to the gaseous state compared to those with smaller molecules.
Smaller molecules tend to have weaker cohesive forces, allowing them to escape the liquid more easily when energy is supplied. This is why lighter substances, such as alcohols, often have higher rates of vaporization compared to heavier liquids like oils or water. The size and structure of the molecules influence how easily they can overcome the attractive forces holding them together within the liquid phase.
Understanding how molecular size affects this process is crucial for applications ranging from industrial distillation to the natural water cycle. By examining the relationship between molecular structure and the rate of change from liquid to gas, we can better predict how substances behave under different conditions.
Practical Applications of Evaporation Studies
Understanding the transition of liquids to gas has wide-ranging implications in both natural processes and industrial applications. The ability to control or predict how quickly a liquid transforms can be essential in areas such as manufacturing, environmental science, and even food preservation. Studies focused on these phenomena help improve processes that rely on the balance between temperature, pressure, and material properties.
Industrial Processes: In industries like chemical engineering, the rate at which liquids vaporize plays a crucial role in designing systems such as distillation columns or cooling towers. By optimizing these processes, manufacturers can enhance energy efficiency, reduce waste, and increase output.
Environmental Impact: Understanding how substances in nature change phases is critical for predicting weather patterns, the water cycle, and even climate change. In ecosystems, the rate of water movement from liquid to gas affects humidity levels, cloud formation, and precipitation.
Food Preservation: In food storage, knowing how moisture escapes from products is key to preventing spoilage. Techniques such as drying or freeze-drying are based on controlling the rate of moisture removal, extending shelf life without compromising quality.
By examining the various factors that influence this phase transition, scientists and engineers can improve technologies, enhance natural resource management, and contribute to more sustainable practices across industries.
Explaining Results in Your Lab Report
When analyzing the outcome of experiments, clear communication of findings is crucial. It’s not enough to simply present data; one must also interpret it in a way that links the results to the underlying theories and hypotheses. The goal is to help others understand the significance of the results, any patterns observed, and how they contribute to the broader understanding of the topic at hand.
Presenting Data Clearly
To make your analysis easy to follow, start by organizing data logically. Use tables or graphs to illustrate key points, making complex information accessible. Ensure that each figure is labeled correctly, with clear captions and units. This allows the reader to understand what is being measured and how the data supports or contradicts the expectations.
Discussing the Significance
Once the data is presented, move on to explaining its meaning. Consider whether the findings align with your hypothesis or whether they suggest an unexpected outcome. Discuss any anomalies or outliers in the data, offering potential explanations. It’s also important to connect the results to existing theories, suggesting how they may support or challenge established ideas.
Drawing Conclusions: The final step in this section is to summarize the implications of the data. What do the findings reveal about the experiment’s objectives? How do they enhance our understanding of the phenomenon being studied? Be sure to provide a well-rounded conclusion, addressing both the strengths and limitations of the experiment.
Suggestions for Future Work: If your results raise new questions or suggest areas for further exploration, highlight these points. Science is an ongoing process, and proposing follow-up studies is an essential part of contributing to the field.